Autonomously active protein kinase C in the maintenance phase of N-methyl-D-aspartate receptor-independent long term potentiation - PubMed (original) (raw)
Comparative Study
. 1994 Nov 11;269(45):27958-63.
Affiliations
- PMID: 7961728
- PMCID: PMC3905312
Comparative Study
Autonomously active protein kinase C in the maintenance phase of N-methyl-D-aspartate receptor-independent long term potentiation
C M Powell et al. J Biol Chem. 1994.
Abstract
In area CA1 of the hippocampus, the induction of long term potentiation (LTP) requires activation of either N-methyl-D-aspartate receptors (NMDA receptor-dependent LTP) or voltage-gated Ca2+ channels (NMDA receptor-independent LTP). We have investigated biochemical sequelae of NMDA receptor-independent LTP induction. We find that a persistent increase in second messenger-independent protein kinase C activity is associated with the maintenance phase of NMDA receptor-independent LTP. This increase in protein kinase C activity is prevented by blocking LTP with nifedipine, a Ca2+ channel antagonist, or kynurenic acid, a nonselective glutamate receptor antagonist. Additionally, we find an increase in the catalytic fragment of protein kinase C (PKM) in the maintenance phase of NMDA receptor-independent LTP, indicating that proteolytic activation of protein kinase C may account for its autonomous activation. This increase in the catalytic fragment of protein kinase C is also prevented by blocking LTP induction. These results are the first to demonstrate that persistent protein kinase C activation is a possible mechanism for the maintenance of NMDA receptor-independent LTP.
Figures
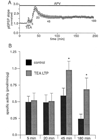
Fig. 1. Basal PKC activity is increased 45 min into the maintenance phase of LTP_K_
A, time course of LTP_K_. pEPSP slope is plotted as a function of time. Each point represents the slope of the average of four consecutive traces normalized to the base-line average of each experiment in this and all subsequent pEPSP time course figures. TEA (25 m
m
) application in the presence of 50 µ
m dl
-2-amino-5-phosphonovaleric acid (APV) caused a lasting potentiation (172 ± 12%, n = 14). B, example pEPSPs taken before (a), during (b), and 45 min after (c) TEA washout. C, increase in basal, Ca2+-independent PKC activity 45 min into LTP_K_ (solid bars). Addition of PKC-(19–36) (5 µ
m
) to kinase assays in vitro blocks the increase in PKC activity associated with LIT_K_ (hatched bars).
Fig. 2. Blocking LTP_K_ with kynurenic acid prevents the increase in PKC activity
A, LTP_K_ was completely blocked when TEA was applied in the presence of 10 m
m
kynurenic acid (n = 5). B, physiological effects of kynurenic acid (10 m
m
) are completely reversible (n = 4). C, no significant increase in PKC activity was observed in slices exposed to TEA in the presence of kynurenic acid. Control slices were similarly perfused with 10 m
m
kynurenic acid.
Fig. 3. Blocking LTP_K_ with 10 µm nifedipine prevents the increase in basal PKC activity
A, time course of pEPSP slope before and after TEA application (25 m
m
) in the presence of 10 µ
m
nifedipine and 50 µ
m
APV. Potentiation is significantly reduced to 108 ± 1% of base line 45 min after TEA washout (n = 5). B, no change in basal PKC activity occurs after TEA application in the presence of 10 µ
m
nifedipine and 50 µ
m
APV (n = 5). Control slices were similarly perfused with nifedipine.
Fig. 4. Time course of the increase in basal PKC activity associated with LTP_K_ maintenance
A, 3-h time course of LTP_K_. pEPSP slope is plotted as a function of time. TEA (25 m
m
) application In the presence of 50 µ
m
APV caused a lasting potentiation (144 ± 6%, n = 7). Inset numbers represent times in minutes where PKC activity was measured in B. B, the increase in PKC activity associated with LTP_K_ persists for at least 3 h following TEA application. Basal PKC activity was measured in separate experiments at various time intervals following onset of TEA washout (5 min, n = 5; 20 min, n = 7; 45 min, n = 14; 180 min, n = 7). A significant increase in basal PKC activity was observed 45 and 180 min following TEA washout (* indicates statistical significance using an unpaired Student's t test, p < 0.05). No significant change in total Ca2+/phosphatidylserine/oleoylacetylglycerol-stimulated PKC activity was observed at any time point (not shown).
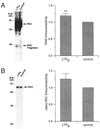
Fig. 5. Proteolytic activation of PKC is associated with the increase in PKC activity
A, a significant increase in the proteolytically activated fragment of PKC occurs 45 min into LTP_K_ maintenance. Left, example Western blot analysis reveals a statistically significant increase in the 45-kDa immunoreactive protein (PKC Fragment) 45 min into LTP_K_ maintenance. Right, grouped densitometry data from LTP_K_ Western blot experiments reveal a statistically significant increase in PKM immunoreactivity (119 ± 6% of control, n = 10, p = 0.009). B, no significant change in native PKC immunoreactivity occurs during LTP_K_. Left, example Western blot analysis (shorter exposure than in A) using a polyclonal antibody raised against classical PKC isoforms demonstrates no change in native PKC immunoreactivity (PKC). Right, grouped densitometry data from LTP_K_ Western blot experiments reveal no significant change in the immunoreactivity of native PKC (n = 10, 45-min time point).
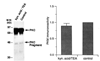
Fig. 6. Blocking LTP_K_ with kynurenic (kyn.) acid prevents the increase in PKM associated with LTP_K_ maintenance
Left, example Western blot analysis using a polyclonal antibody raised against classical PKC isoforms demonstrates no significant increase in PKM when LTP_K_ is blocked by kynurenic acid. Right, grouped densitometry data from kynurenic acid block experiments show no increase in PKM when LTP_K_ is blocked by kynurenic acid (89 ± 8% of control, n = 6).
Similar articles
- Evidence for postsynaptic induction and expression of NMDA receptor independent LTP.
Grover LM. Grover LM. J Neurophysiol. 1998 Mar;79(3):1167-82. doi: 10.1152/jn.1998.79.3.1167. J Neurophysiol. 1998. PMID: 9497399 - Activation of pre- and postsynaptic protein kinase C during tetraethylammonium-induced long-term potentiation in the CA1 field of the hippocampus.
Ramakers GM, Pasinelli P, van Beest M, van der Slot A, Gispen WH, De Graan PN. Ramakers GM, et al. Neurosci Lett. 2000 May 26;286(1):53-6. doi: 10.1016/s0304-3940(00)01081-8. Neurosci Lett. 2000. PMID: 10822151 - [Long-term potentiation of the NMDA-dependent component of the EPSP in the hippocampus].
Kleshchevnikov AM. Kleshchevnikov AM. Usp Fiziol Nauk. 1998 Oct-Dec;29(4):6-23. Usp Fiziol Nauk. 1998. PMID: 9883495 Review. Russian. - Long-term potentiation and the role of N-methyl-D-aspartate receptors.
Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL. Volianskis A, et al. Brain Res. 2015 Sep 24;1621:5-16. doi: 10.1016/j.brainres.2015.01.016. Epub 2015 Jan 22. Brain Res. 2015. PMID: 25619552 Free PMC article. Review.
Cited by
- Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C.
Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ. Sutton MA, et al. J Neurosci. 2004 Apr 7;24(14):3600-9. doi: 10.1523/JNEUROSCI.1134-03.2004. J Neurosci. 2004. PMID: 15071108 Free PMC article. - Induction of a specific olfactory memory leads to a long-lasting activation of protein kinase C in the antennal lobe of the honeybee.
Grünbaum L, Müller U. Grünbaum L, et al. J Neurosci. 1998 Jun 1;18(11):4384-92. doi: 10.1523/JNEUROSCI.18-11-04384.1998. J Neurosci. 1998. PMID: 9592115 Free PMC article. - RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes.
Gerendasy DD, Sutcliffe JG. Gerendasy DD, et al. Mol Neurobiol. 1997 Oct;15(2):131-63. doi: 10.1007/BF02740632. Mol Neurobiol. 1997. PMID: 9396008 Review. - Dendritic transport and localization of protein kinase Mzeta mRNA: implications for molecular memory consolidation.
Muslimov IA, Nimmrich V, Hernandez AI, Tcherepanov A, Sacktor TC, Tiedge H. Muslimov IA, et al. J Biol Chem. 2004 Dec 10;279(50):52613-22. doi: 10.1074/jbc.M409240200. Epub 2004 Sep 15. J Biol Chem. 2004. PMID: 15371429 Free PMC article. - Learning-induced glutamate receptor phosphorylation resembles that induced by long term potentiation.
Shukla K, Kim J, Blundell J, Powell CM. Shukla K, et al. J Biol Chem. 2007 Jun 22;282(25):18100-18107. doi: 10.1074/jbc.M702906200. Epub 2007 May 1. J Biol Chem. 2007. PMID: 17472959 Free PMC article.
References
- Johnston D, Williams S, Jaffe D, Gray R. Annu. Rev. Physiol. 1992;54:489–505. - PubMed
- Grover LM, Teyler TJ. Nature. 1990;347:477–479. - PubMed
- Aniksztejn L, Ben-Ari Y. Nature. 1991;349:67–69. - PubMed
- Madison DV, Malenka RC, Nicoll RA. Annv. Rev. Neurosci. 1991;14:379–397. - PubMed
- Harris EW, Ganong AH, Cotman CW. Brain Res. 1984;323:132–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH48186/MH/NIMH NIH HHS/United States
- MH48432/MH/NIMH NIH HHS/United States
- F30 MH010338-03/MH/NIMH NIH HHS/United States
- R37 MH044754/MH/NIMH NIH HHS/United States
- R01 MH048432/MH/NIMH NIH HHS/United States
- MH44754/MH/NIMH NIH HHS/United States
- F30 MH010338/MH/NIMH NIH HHS/United States
- F30 MH010338-04/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous