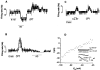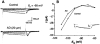Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal - PubMed (original) (raw)
Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal
D G Rainnie et al. Science. 1994.
Erratum in
- Science 1994 Jul 1;265(5168):16
Abstract
Increased discharge activity of mesopontine cholinergic neurons participates in the production of electroencephalographic (EEG) arousal; such arousal diminishes as a function of the duration of prior wakefulness or of brain hyperthermia. Whole-cell and extracellular recordings in a brainstem slice show that mesopontine cholinergic neurons are under the tonic inhibitory control of endogenous adenosine, a neuromodulator released during brain metabolism. This inhibitory tone is mediated postsynaptically by an inwardly rectifying potassium conductance and by an inhibition of the hyperpolarization-activated current. These data provide a coupling mechanism linking neuronal control of EEG arousal with the effects of prior wakefulness, brain hyperthermia, and the use of the adenosine receptor blockers caffeine and theophylline.
Figures
Fig. 1
Endogenous AD exerts a tonic inhibition in the LDT and DBB, in vitro. (A) Spike frequency histogram of extracellularly recorded action potential firing in a neuron of the LDT. Superfusion with AD (100 μM) causes a marked reduction of firing frequency and CPT (10 μM) causes a prolonged increase in firing frequency. (B) In the DBB, application of CPT (10 μM) similarly increases firing rates, and subsequent exogenous AD decreased firing rates. (C) Application of 8-_p_-ST (50 μM) mimics the effect of CPT in an LDT neuron. (D) Whole-cell voltage-clamp recording of the response of a histochemically identified LDT cholinergic neuron to OPT application. _E_m, membrane potential. Digital subtraction of steady-state voltage-current relations obtained before and during OPT (10 μM) reveals a CPT-induced current with voltage and kinetic characteristics of _I_h. (Inset) Enhanced inward relaxation during CPT application. The relatively hyperpolarized reversal potential for _I_h may reflect a small additional presynaptic input evoked by CPT.
Fig. 2
Exogenous AD application reduces an inwardly relaxing _I_h current in LDT neurons. (A) Current traces of an LDT neuron before and during AD application. Voltage step commands (−10 to −50 mV; 500 ms) from a holding potential of −60 mV reveal a slow inwardly relaxing current of increasing amplitude (upper traces). The presence of AD (20 μM) reduces the expression of the inward relaxation (lower traces). (B) A plot of the voltage-current relation determined for the inward relaxation before and during AD application. (Inset) The inward relaxation current (_I_relax) was calculated by subtraction of the instantaneous current (_I_i) from the steady-state current (_I_ss) for each of the current traces in (A).
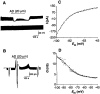
Fig. 3
Exogenous AD application evokes a membrane hyperpolarization mediated by activation of an inwardly rectifying K+ conductance in neurons of the LDT. (A) In a whole-cell current-clamp recording, AD evokes a membrane hyperpolarization and an associated decrease in membrane input resistance. (B) Current trace from another neuron voltage clamped at _V_h = −60 mV. Application of AD evokes an outward current and an associated increase in membrane conductance. Downward deflections in (A) and (B) reflect the voltage and current response to 100-pA, 20-mV hyperpolarizing step commands 200 ms in duration that were used to determine the resistance and conductance. (C) A plot of AD current as a function of membrane potential reveals AD activation of an inwardly rectifying K+ conductance. AD current was calculated by digital subtraction of the current evoked by “ramping” the neuron from −100 to −40 mV [see (B)] in control from that obtained during AD application. (D) AD chord conductance G as a function of membrane potential is well fit by a Boltzmann equation (dashed line) with a half-activation potential _V_1/2 of −85 mV and a slope factor of k = 9 [same neuron as in (C)].
Similar articles
- Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state.
Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Strecker RE, et al. Behav Brain Res. 2000 Nov;115(2):183-204. doi: 10.1016/s0166-4328(00)00258-8. Behav Brain Res. 2000. PMID: 11000420 - Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study.
Leonard CS, Llinás R. Leonard CS, et al. Neuroscience. 1994 Mar;59(2):309-30. doi: 10.1016/0306-4522(94)90599-1. Neuroscience. 1994. PMID: 8008195 - Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems.
Steriade M, Datta S, Paré D, Oakson G, Curró Dossi RC. Steriade M, et al. J Neurosci. 1990 Aug;10(8):2541-59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. J Neurosci. 1990. PMID: 2388079 Free PMC article. - Discharge patterns of neurons in cholinergic regions of the basal forebrain during waking and sleep.
Szymusiak R, Alam N, McGinty D. Szymusiak R, et al. Behav Brain Res. 2000 Nov;115(2):171-82. doi: 10.1016/s0166-4328(00)00257-6. Behav Brain Res. 2000. PMID: 11000419 Review. - The cholinergic system and EEG slow waves.
Riekkinen P, Buzsaki G, Riekkinen P Jr, Soininen H, Partanen J. Riekkinen P, et al. Electroencephalogr Clin Neurophysiol. 1991 Feb;78(2):89-96. doi: 10.1016/0013-4694(91)90107-f. Electroencephalogr Clin Neurophysiol. 1991. PMID: 1704840 Review. No abstract available.
Cited by
- Paeoniflorin exerts analgesic and hypnotic effects via adenosine A1 receptors in a mouse neuropathic pain model.
Yin D, Liu YY, Wang TX, Hu ZZ, Qu WM, Chen JF, Cheng NN, Huang ZL. Yin D, et al. Psychopharmacology (Berl). 2016 Jan;233(2):281-93. doi: 10.1007/s00213-015-4108-6. Epub 2015 Oct 29. Psychopharmacology (Berl). 2016. PMID: 26514553 - Sleep need driven oscillation of glutamate synaptic phenotype.
Vogt KE, Kulkarni A, Pandey R, Dehnad M, Konopka G, Greene RW. Vogt KE, et al. Elife. 2025 Feb 14;13:RP98280. doi: 10.7554/eLife.98280. Elife. 2025. PMID: 39950545 Free PMC article. - Modulatory effects of adenosine on inhibitory postsynaptic potentials in the lateral amygdala of the rat.
Heinbockel T, Pape HC. Heinbockel T, et al. Br J Pharmacol. 1999 Sep;128(1):190-6. doi: 10.1038/sj.bjp.0702761. Br J Pharmacol. 1999. PMID: 10498851 Free PMC article. - A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain.
Thakkar MM, Winston S, McCarley RW. Thakkar MM, et al. J Neurosci. 2003 May 15;23(10):4278-87. doi: 10.1523/JNEUROSCI.23-10-04278.2003. J Neurosci. 2003. PMID: 12764116 Free PMC article. - Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis.
Alam MN, Szymusiak R, Gong H, King J, McGinty D. Alam MN, et al. J Physiol. 1999 Dec 15;521 Pt 3(Pt 3):679-90. doi: 10.1111/j.1469-7793.1999.00679.x. J Physiol. 1999. PMID: 10601498 Free PMC article.
References
- The behavioral effects of caffeine and theophylline seem to derive from their activity as adenosine antagonists [inhibition constant _K_l < 60 μM for the A1 and A2 receptors; Sattin A, Rall TW. Mol. Pharmacol. 1970;6:13.; Ponjs F, Bruns F, Daly JW. J. Neurochem. 1980;34:1319. Snyder SH. Annu. Rev. Neurosci. 1985;8:103. rather than their activity as either phosphodiesterase inhibitors [constant for 50% inhibition IC.50 > 350 μM; Choi OH, Shamin MT, Padgett WL, Daly JW. Life Sci. 1988;43:387. or mediators of intracellular calcium release [median effective concentration EC50 = 6 mM; McPherson PS, et al. Neuron. 1991;7:17. Kuba K. J. Physiol. 1980;298:547. Neering IR, McBurney RN. Nature. 1984;309:158. Freil DD, Tsien RW. J. Physiol. 1992;450:217.
- Horne J. Experientia. 1992;48:941. - PubMed
- McGinty D, Szymusiak R. Trends Neurosci. 1990;13:480. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources