The dynamics of dendritic structure in developing hippocampal slices - PubMed (original) (raw)
The dynamics of dendritic structure in developing hippocampal slices
M E Dailey et al. J Neurosci. 1996.
Abstract
Time-lapse fluorescence confocal microscopy was used to directly visualize the formation and dynamics of postsynaptic target structures (i.e., dendritic branches and spines) on pyramidal neurons within developing tissue slices. Within a 2 week period of time, pyramidal neurons in cultured slices derived from early postnatal rat (postnatal days 2-7) developed complex dendritic arbors bearing numerous postsynaptic spines. At early stages (1-2 d in vitro), many fine filopodial protrusions on dendrite shafts rapidly extended (maximum rate approximately 2.5 microM/minute) and retracted (median filopodial lifetime, 10 min), but some filopodia transformed into growth cones and nascent dendrite branches. As dendritic arbors matured, the population of fleeting lateral filopodia was replaced by spine-like structures having a low rate of turnover. This developmental progression involved a transitional stage in which dendrites were dominated by persistent (up to 22 hr) but dynamic spiny protrusions (i.e., protospines) that showed substantial changes in length and shape on a timescale of minutes. These observations reveal a highly dynamic state of postsynaptic target structures that may actively contribute to the formation and plasticity of synaptic connections during CNS development.
Figures
Fig. 1.
Dendrites of pyramidal cells grow and differentiate in in vitro tissue slices. The structure of a differentiated hippocampal CA1 pyramidal neuron is revealed by postfixation labeling with DiI after 2 weeks in slice culture.A, Low-magnification confocal image showing characteristic polarized pyramidal shape. Emerging from the cell soma (arrow) are several basal dendrites and a thick apical dendrite with several oblique secondary branches (arrowheads). B, Higher-magnification view of distal apical dendrites within the stratum radiatum showing secondary branches. Although their full extent is not evident in this single confocal image plane, these branches course throughout the thickness of the tissue slice. C, Dendritic branches are studded with numerous spine-like projections (arrowheads), 1–3 μm in length, presumed to be sites of synaptic contact. These features are characteristic of pyramidal neurons in vivo.
Fig. 5.
Dynamics of spine-like protrusions on well developed pyramidal cell dendrites (12 d in vitro).A, Single-focal-plane view of a CA1 neuron showing the characteristic pyramidal shape with a primary apical dendrite (arrow). The tissue was prepared from a P6 animal and cultured for 12 d. B, Higher-magnification, extended-focus view (combining 6 optical sections spanning 30 μm) of the distal portion of the apical dendrites. Note the complexity of the dendritic arbor and spiny appearance of the oblique dendrite branches (arrows). C, Time-lapse sequence of a region (top box) in B showing both stable, spine-like structures and transient filopodial extensions. A short, knobby spine-like protrusion (arrowhead 1) persists with little change in length or shape. Directly adjacent to this spine, a filopodia-like protrusion (arrowhead 2) extends transiently from the dendrite shaft. Time is shown in minutes. D, Time-lapse sequence of a region (lower box) in_B_ showing rapid formation of a knobby, spine-like protrusion (arrowhead 5). The new spine forms and persists adjacent to an existing spine (arrowhead 4). Note also another spine (arrowhead 3) that persists for the entire observation period but does not change shape or length significantly. Scale bar in_C_ also applies to D. The plots of length versus time for spiny protrusions 1, 2, and 5 correspond to traces 12, 9, and 11, respectively, in Figure 7.
Fig. 6.
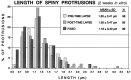
Distribution of spine lengths in fixed or live (time-lapse imaged) slices (2 weeks in vitro). The lengths of all spiny protrusions on dendrites in a live slice were determined at the beginning (PRE-TIME LAPSE) and end (POST-TIME LAPSE) of a standard 3 hr time-lapse imaging sequence (experiment shown in Fig. 5). There was no significant difference in the mean length or length distribution of spines before or after the imaging session, indicating that the time-lapse imaging did not induce changes in spine structure. Also, the mean length of spines on live cells was very similar to the distribution of spine lengths on dendrites in comparable slices fixed before labeling and imaging (FIXED). See Results for more details.
Fig. 8.
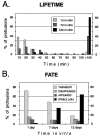
Stage-dependent differences in the lifetime and fate of spiny protrusions. A, The lifetimes of spiny protrusions increased progressively with time in vitro. Distribution of lifetimes of 146 spiny protrusions from apical dendrites of eight pyramidal cells in six tissue slices. B, Fate of spiny protrusions in tissue slices. All spiny structures observed during the first 3 hr of imaging were classified as_TRANSIENT_ (came and went during the 3 hr observation period), DISAPPEARED (evident at the beginning but were resorbed during the 3 hr observation), APPEARED (formed_de novo_ and persisted), or STABLE (evident throughout the 3 hr observation period). Note that a greater fraction of spines were stable on more mature (i.e., 12 d in vitro) dendrites, but there remained a low level of spine turnover.
Fig. 2.
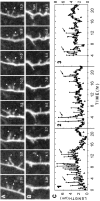
Sprouting of lateral filopodia, growth cones, and collateral branches from pyramidal cell dendrites during early stages of differentiation (1 d in vitro). A, Low-magnification image of DiI-labeled CA1 pyramidal neuron somata (P) and dendrites (arrows) in a live tissue slice taken from a P2 animal. This is an extended-focus image made by combining a stack of five optical sections spanning 15 μm in the_Z_-dimension. At early developmental stages, dendrites are often tipped by complex growth cones (arrowhead).SR, Stratum radiatum; SP, stratum pyramidale.B, Through-focus sequence of boxed _region_in A showing a segment of an apical dendrite shaft (arrow) having fine, lateral filopodial protrusions (arrowhead). For time-lapse imaging, multiple optical sections were collected to ensure that small dendritic protrusions were captured in their entirety. C, Time-lapse sequence of the same field as in B showing that numerous lateral filopodia (arrowheads) extend from, and retract back to, the dendrite shaft. Most filopodia are resorbed within a few minutes after first appearing. These images are composites of two or three optical sections from the middle of a five-image stack (shown in B).D, Persisting lateral filopodia sometimes evolve into growth cones. Time-lapse sequence shows a lateral filopodium (arrowheads) sprouting from an apical dendrite shaft (0–30 min), persisting for more than an hour (30–90 min), then rapidly developing into a complex, growth cone-like structure (arrow; 90–100 min). E,De novo sprouting of a growth cone leading to formation of a collateral dendrite branch. A faint, filopodial structure is seen first (40 min), then a growth cone develops (60–80 min) and spins out a new branch that persisted and grew to a length of at least 35 μm (120 min). For C_–_E, elapsed time is shown in minutes.
Fig. 3.
Dynamics of spiny protrusions on differentiating pyramidal cell dendrites (7 d in vitro). Tissue was taken from a P5 rat and cultured for 1 week. A, Low-magnification image of a live, DiI-labeled CA1 pyramidal cell (arrow) showing extensive apical dendrite arbors that have developed in vitro. This image is a Z_-axis composite of 15 separate confocal images spanning a tissue depth of 45 μm. Because of the greater volume of tissue sampled, dendritic arbors appear more complex in Z_-axis composite images than in single focal plane images. The complexity of dendritic arbors at 7 d in vitro was intermediate to that of neurons at 1 d (compare Fig.2_A) and 12 d (compare Figs. 1_A, 5_A_)in vitro. B, Higher-magnification image of boxed region in A showing many simple and branched (arrows) filopodia as well as spine-like protrusions with bulbous tips (arrowheads). Schaffer axons from CA3 pyramidal cells, which are retained in the tissue slices, normally synaptically terminate on spines in this region of the dendrite. Scale bar in_B_ applies to B–D. C, Selected images of a time-lapse sequence of a region in B (right box) showing extension and partial retraction of a filopodium (arrow). By contrast, the length of a spine-like protrusion with a bulbous tip (arrowhead) is stable over the same time period. D, Time-lapse sequence of a region in_B_ (left box) showing dynamic changes in structure of a spine-like protrusion. The protrusion starts with a bulbous head (arrow) that appears to bifurcate (arrowheads). Images in C and D, shown at 1 min intervals, are single-focal-plane scans selected from an original data set taken at 30 sec intervals.
Fig. 4.
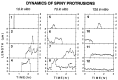
Dynamics of long-lived, spiny protrusions on differentiating dendrites (7 d in vitro). These extended time-lapse sequences show changes in length and shape of spiny protrusions (arrowheads) over a period of up to 18.6 hr. All images are composites of two adjacent focal planes selected from a five-image stack to ensure that the full lengths of the structures were captured. Images were selected from an original data set collected at 5 min intervals. Elapsed time is shown in hours. A, Two of the spiny protrusions (1 and 2) were highly dynamic but persisted for the entire observation period. Length plots for these protrusions are shown in C (traces 1 and_2_). B, De novo appearance of a spiny protrusion (arrowhead) that persisted after being formed. Length plot for this protrusion is shown in C (trace 3). C, Plots of length versus time for spiny protrusions shown above. Measurements of protrusion length were made at each time point (5 min intervals). Traces 1 and_2_ correspond to the protrusions indicated in A. Note that transient length excursions (arrows), which can effectively double or triple the length of the protrusion, are made from relatively stable bases (arrowheads). Reduction in the transient length excursions of protrusions 1 and_3_ near the end of the sequences suggests a further stabilization of these protrusions. In contrast, the transient length excursions persist in trace 2.
Fig. 7.
Stage-dependent differences in the dynamics of spiny protrusions. Representative plots of length versus time for spiny dendritic protrusions at 1 d (traces 1–4), 7 d (traces 5–8), and 12 d (traces 9–12) in vitro. The traces illustrate a range of behaviors, including transient protrusion (1, 2, 4, 5, 9), resorption of preexisting structures (6, 10), de novo formation and persistence (3, 7, 11), and persistence of preexisting structures (8, 12). Transient filopodia-like protrusions (1, 2, 5, 9) were observed at all times in vitro_but were most prominent at early stages (i.e., 1 d in vitro). At 7 d in vitro, persisting spiny protrusions often showed transient length excursions (arrows) from short, stable bases (arrowheads) (compare trace 8 and Fig. 4_C). Most persisting spines at 12 d in vitro showed little change in length (e.g., trace 12), although there was some turnover of spines (e.g., traces 10, 11). (Note different time base of Figs. 4_C_ and7.)
Similar articles
- Age-dependence in the homeostatic upregulation of hippocampal dendritic spine number during blocked synaptic transmission.
Kirov SA, Goddard CA, Harris KM. Kirov SA, et al. Neuropharmacology. 2004 Oct;47(5):640-8. doi: 10.1016/j.neuropharm.2004.07.039. Neuropharmacology. 2004. PMID: 15458835 - Rapid formation and remodeling of postsynaptic densities in developing dendrites.
Marrs GS, Green SH, Dailey ME. Marrs GS, et al. Nat Neurosci. 2001 Oct;4(10):1006-13. doi: 10.1038/nn717. Nat Neurosci. 2001. PMID: 11574832 - Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia.
Portera-Cailliau C, Pan DT, Yuste R. Portera-Cailliau C, et al. J Neurosci. 2003 Aug 6;23(18):7129-42. doi: 10.1523/JNEUROSCI.23-18-07129.2003. J Neurosci. 2003. PMID: 12904473 Free PMC article. - [On the function of dendritic filopodia].
Portera Cailliau C, Yuste R. Portera Cailliau C, et al. Rev Neurol. 2001 Dec 16-31;33(12):1158-66. Rev Neurol. 2001. PMID: 11785056 Review. Spanish. - Ca2+ signalling in postsynaptic dendrites and spines of mammalian neurons in brain slice.
Müller W, Connor JA. Müller W, et al. J Physiol Paris. 1992;86(1-3):57-66. doi: 10.1016/s0928-4257(05)80008-7. J Physiol Paris. 1992. PMID: 1343597 Review.
Cited by
- Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal area CA1 of the rat.
Cao G, Harris KM. Cao G, et al. J Neurophysiol. 2012 Feb;107(3):902-12. doi: 10.1152/jn.00780.2011. Epub 2011 Nov 23. J Neurophysiol. 2012. PMID: 22114158 Free PMC article. - Rapid hippocampal network adaptation to recurring synchronous activity--a role for calcineurin.
Casanova JR, Nishimura M, Le J, Lam TT, Swann JW. Casanova JR, et al. Eur J Neurosci. 2013 Oct;38(8):3115-27. doi: 10.1111/ejn.12315. Epub 2013 Jul 24. Eur J Neurosci. 2013. PMID: 23879713 Free PMC article. - Sampling issues in quantitative analysis of dendritic spines morphology.
Ruszczycki B, Szepesi Z, Wilczynski GM, Bijata M, Kalita K, Kaczmarek L, Wlodarczyk J. Ruszczycki B, et al. BMC Bioinformatics. 2012 Aug 25;13:213. doi: 10.1186/1471-2105-13-213. BMC Bioinformatics. 2012. PMID: 22920322 Free PMC article. - Divergent roles of p75NTR and Trk receptors in BDNF's effects on dendritic spine density and morphology.
Chapleau CA, Pozzo-Miller L. Chapleau CA, et al. Neural Plast. 2012;2012:578057. doi: 10.1155/2012/578057. Epub 2012 Mar 27. Neural Plast. 2012. PMID: 22548193 Free PMC article. - The origin recognition core complex regulates dendrite and spine development in postmitotic neurons.
Huang Z, Zang K, Reichardt LF. Huang Z, et al. J Cell Biol. 2005 Aug 15;170(4):527-35. doi: 10.1083/jcb.200505075. Epub 2005 Aug 8. J Cell Biol. 2005. PMID: 16087709 Free PMC article.
References
- Amaral D, Dent JA. Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
- Andersen P. Factors influencing functional connectivity during hippocampal development. Prog Brain Res. 1979;51:139–147. - PubMed
- Bayer SA. The development of the hippocampal region in the rat. I. Neurogenesis examined with [3H]thymidine autoradiography. J Comp Neurol. 1980;190:87–114. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials