Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons - PubMed (original) (raw)
Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons
A M Craig et al. J Neurosci. 1996.
Abstract
The molecular mechanisms underlying the establishment of a postsynaptic receptor mosaic on CNS neurons are poorly understood. One protein thought to be involved is gephyrin, a peripheral membrane protein that binds to the inhibitory glycine receptor and functions in clustering this receptor at synapses in cultured rat spinal cord neurons. We investigated the possible association of gephyrin with synapses in cultured rat hippocampal neurons, where glutamate and GABA but not glycine are the principal transmitters. Gephyrin immunoreactivity was detected in axons as well as dendrites, changing from a predominantly axonal to a more dendritic distribution with time in culture. Gephyrin staining was not distributed uniformly, but always took the form of clusters. Small clusters of gephyrin (0.2 microns 2), present throughout development, were distributed widely and not restricted to synaptic sites. Larger clusters of gephyrin (0.4-10.0 microns 2, sometimes composed of groups of small clusters), which developed in older cells, were localized to a subset of contacts between axons and dendrites. These large clusters were not present at glutamatergic synapses (marked by immunostaining for GluR1), but were closely associated with GABAergic synapses (marked by immunostaining for GABA and glutamic acid decarboxylase). These results, together with previous findings, suggest that gephyrin may function to anchor GABA and glycine receptors, but not glutamate receptors, at postsynaptic sites on central neurons. They also raise the possibility that gephyrin has additional functions, independent of its role at synapses.
Figures
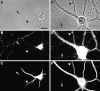
Fig. 1.
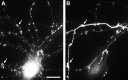
Segregation of gephyrin first to axons and then to dendrites during the development of hippocampal neurons in culture. Neurons were double-labeled for gephyrin (B, E) and the dendritic protein MAP2 (C, F) after 8 d (A–C) or 22 d (D–F) in culture. Phase-contrast micrographs (A, D) reveal the increase in complexity of the axonal and dendritic network. In the younger neurons, gephyrin immunoreactivity was strong in axons (arrow) but undetectable in dendrites (arrowhead). In the more mature neurons, gephyrin was restricted mainly to dendrites (arrowhead) but still detectable in dispersed puncta in a small subset of axons (arrow). The presence of gephyrin immunoreactivity in axons was confirmed in additional experiments by double-label immunocytochemistry for neurofilament 200 (data not shown). Gephyrin immunoreactivity was always detected in a punctate pattern. Scale bar (shown in A): 20 μm.
Fig. 2.
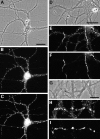
Development of large gephyrin clusters on dendrites. Hippocampal neurons were immunolabeled after 12 d (A, B) or 16 d (C, D) in culture for gephyrin (A, C) and the glutamate receptor subunit GluR1 (B, D). GluR1 is segregated to dendrites and has begun to form postsynaptic clusters on spines. At 12 d, gephyrin was present in both axons (arrow) and dendrites. In dendrites, in addition to the small clusters, a few large gephyrin clusters had formed (arrowhead). By 16 d, axonal labeling had decreased but was still detectable in spots (arrow), and the number of large dendritic clusters had increased (arrowhead). Scale bars (shown in A and C): 10 μm.
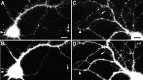
Fig. 5.
Absence of a relationship between gephyrin clusters and GluR1-labeled glutamate synapses. Hippocampal neurons after 3 weeks in culture were immunolabeled for gephyrin (A, C) and the glutamate receptor subunit GluR1 (B, D). Large gephyrin clusters (arrow) did not colocalize with GluR1 clusters (arrowhead). The large gephyrin clusters were always present on dendritic shafts, whereas GluR1 clusters were predominantly on dendritic spines. A minority of GluR1-labeled spines contained small clusters of gephyrin, but the majority of GluR1-labeled synapses contained no detectable gephyrin and the majority of small gephyrin clusters did not colocalize with GluR1 clusters. Scale bars (shown in A and C): 10 μm.
Fig. 6.
Similar distribution patterns of gephyrin and the GABAA receptor β2/3 subunits on different pyramidal cells. Hippocampal neurons after 3 weeks in culture were labeled for either gephyrin (A, B) or GABAA receptor β2/3 subunits (C, D). This was not a double-label experiment, because these two antibodies are from the same species. The overall distribution patterns were similar for both proteins in that large clusters of both proteins were of roughly the same size, were elongated in shape, and were present on dendritic shafts but not on spines. There were additional small clusters of gephyrin that did not have a GABA receptor counterpart. Although it may not be apparent here, the diffuse dendritic staining was generally higher for the GABA receptor subunits than for gephyrin. Scale bars: A, C, 20 μm; B,D, 10 μm.
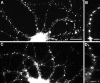
Fig. 7.
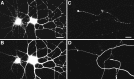
Selective clustering of gephyrin at sites of contact between dendrites and GABAergic axons. Hippocampal neurons after 3 weeks in culture were labeled for gephyrin (A) and GABA (B). Gephyrin was detected in small clusters throughout the soma and dendrites and in larger clusters, or closely spaced groups of small brighter clusters, at points of contact with GABA axons (arrows). The cell shown is a pyramidal cell; the GABAergic axon originated from a nearby interneuron. Scale bar (shown in_A_): 10 μm.
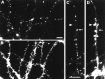
Fig. 8.
Selective clustering of gephyrin at GABAergic synapses. Hippocampal neurons after 3–4 weeks in culture were immunolabeled for gephyrin (B, E, H) and GAD, a marker of GABAergic terminals (C, F, I). There was a largely one-to-one correspondence between large gephyrin clusters and GAD-labeled boutons. Presynaptic versus postsynaptic sites were not distinguishable at this level of resolution, and so the apparent colocalization is consistent with apposition of presynaptic GAD and presumably postsynaptic gephyrin. Smaller gephyrin clusters, most prominent in H, did not colocalize with GAD. The phase-contrast micrographs (A, D, G) indicate the presence of many unlabeled axons and regions of dendrites. The cells shown here are all pyramidal cells; the GABAergic axons originated from nearby interneurons. Scale bars: A–F, 20 μm; G–I, 10 μm.
Fig. 9.
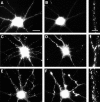
Correlation of gephyrin cluster size with GABAergic innervation. Hippocampal neurons after 24 d in culture were labeled for gephyrin (A, C, E) and GABA (B, D, F). Panels on the right show an enlargement of gephyrin staining in one of the dendrites. Gephyrin cluster size was uniformly small in dendrites lacking GABAergic innervation (A, B). Innervated dendrites exhibited either very large clusters (C, D), or more commonly, both large and small clusters (E, F). The cells shown here are all pyramidal cells from a single coverslip; the GABA-staining in the cell bodies is background because of the greater thickness of the cell bodies. Scale bars:A–F, 20 μm; right panels, 5 μm.
Fig. 3.
Gephyrin expression by both pyramidal and GABAergic neurons. Hippocampal neurons were immunolabeled after 21–24 d in culture for gephyrin (A, C) and GABA (B, D). Gephyrin immunoreactivity was detected in a punctate dendritic pattern in both pyramidal neurons (cell at left in A, B) and GABAergic interneurons (cell at right in A, B). The apparent staining of the pyramidal cell soma by GABA is background that is attributable to the greater thickness of the cell body. The fine-staining of processes surrounding the pyramidal cell soma is typical of GABA axon staining. The minority of axons exhibiting gephyrin immunoreactivity in mature cultures (C) generally did not correspond to GABA axons (D). As in the example shown, gephyrin immunoreactivity in axons of older neurons was typically strongest at the tip. Scale bars: A, B, 30 μm; C, D, 10 μm.
Fig. 4.
Nonsynaptic localization of small gephyrin clusters. Hippocampal neurons after 2 weeks in culture were immunolabeled for gephyrin (B) and the synaptic vesicle protein synaptophysin (C). Only one elongated gephyrin cluster (arrowhead) exhibited a clear synaptic localization evidenced by colocalization with synaptophysin. The vast majority of the small round gephyrin clusters were present at nonsynaptic sites (arrows), and the vast majority of synaptic vesicle clusters contained no detectable gephyrin. These conclusions were confirmed by superposition of color images (not shown). Scale bar (shown in_A_): 10 μm.
Similar articles
- Differential regulation of GABA(A) receptor and gephyrin postsynaptic clustering in immature hippocampal neuronal cultures.
Studler B, Sidler C, Fritschy JM. Studler B, et al. J Comp Neurol. 2005 Apr 11;484(3):344-55. doi: 10.1002/cne.20472. J Comp Neurol. 2005. PMID: 15739236 - GABAergic and glutamatergic terminals differentially influence the organization of GABAergic synapses in rat cerebellar granule cells in vitro.
Studler B, Fritschy JM, Brünig I. Studler B, et al. Neuroscience. 2002;114(1):123-33. doi: 10.1016/s0306-4522(02)00206-3. Neuroscience. 2002. PMID: 12207960 - GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABA(A) receptors and gephyrin.
Brünig I, Suter A, Knuesel I, Lüscher B, Fritschy JM. Brünig I, et al. J Neurosci. 2002 Jun 15;22(12):4805-13. doi: 10.1523/JNEUROSCI.22-12-04805.2002. J Neurosci. 2002. PMID: 12077177 Free PMC article. - Gephyrin: where do we stand, where do we go?
Fritschy JM, Harvey RJ, Schwarz G. Fritschy JM, et al. Trends Neurosci. 2008 May;31(5):257-64. doi: 10.1016/j.tins.2008.02.006. Epub 2008 Apr 9. Trends Neurosci. 2008. PMID: 18403029 Review. - Gephyrin: a master regulator of neuronal function?
Tyagarajan SK, Fritschy JM. Tyagarajan SK, et al. Nat Rev Neurosci. 2014 Mar;15(3):141-56. doi: 10.1038/nrn3670. Nat Rev Neurosci. 2014. PMID: 24552784 Review.
Cited by
- Fmrp regulates neuronal balance in embryonic motor circuit formation.
Barker CM, Miles KD, Doll CA. Barker CM, et al. Front Neurosci. 2022 Nov 3;16:962901. doi: 10.3389/fnins.2022.962901. eCollection 2022. Front Neurosci. 2022. PMID: 36408418 Free PMC article. - GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons.
Christie SB, Miralles CP, De Blas AL. Christie SB, et al. J Neurosci. 2002 Feb 1;22(3):684-97. doi: 10.1523/JNEUROSCI.22-03-00684.2002. J Neurosci. 2002. PMID: 11826098 Free PMC article. - Transient directed motions of GABA(A) receptors in growth cones detected by a speed correlation index.
Bouzigues C, Dahan M. Bouzigues C, et al. Biophys J. 2007 Jan 15;92(2):654-60. doi: 10.1529/biophysj.106.094524. Epub 2006 Oct 27. Biophys J. 2007. PMID: 17071660 Free PMC article. - Nano-organization of spontaneous GABAergic transmission directs its autonomous function in neuronal signaling.
Guzikowski NJ, Kavalali ET. Guzikowski NJ, et al. Cell Rep. 2022 Aug 9;40(6):111172. doi: 10.1016/j.celrep.2022.111172. Cell Rep. 2022. PMID: 35947950 Free PMC article. - Incremental conductance levels of GABAA receptors in dopaminergic neurones of the rat substantia nigra pars compacta.
Guyon A, Laurent S, Paupardin-Tritsch D, Rossier J, Eugène D. Guyon A, et al. J Physiol. 1999 May 1;516 ( Pt 3)(Pt 3):719-37. doi: 10.1111/j.1469-7793.1999.0719u.x. J Physiol. 1999. PMID: 10200421 Free PMC article.
References
- Apel ED, Roberds SL, Campbell KP, Merlie JP (1995) Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron 15: 115–126. - PubMed
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
- Benson DL, Watkins FH, Steward O, Banker G. Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol. 1994;23:279–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources