Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability - PubMed (original) (raw)
Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability
L Brundage et al. Neuron. 1996 May.
Abstract
We find that C. elegans egl-30 encodes a heterotrimeric G protein a subunit more than 80% identical to mammalian Gqalpha family proteins, and which can function as a Gqalpha subunit in COS-7 cells. We have identified new egl-30 alleles in a selection for genes involved in the C. elegans acetylcholine response. Two egl-30 alleles specify premature termination of Gqalpha and are essentially lethal in homozygotes. Animals homozygous for six other egl-30 alleles are viable and fertile, but exhibit delayed egg laying and leave flattened tracks. Overexpression of the wild-type egl-30 gene produces the opposite behavior. Analysis of these mutants suggest that their phenotypes reflect defects in the muscle or neuromuscular junction.
Figures
Figure 1. A C. elegans Homolog of Gqα and G11α
(A) The deduced amino acid sequence of C. elegans Gqα is shown compared with Gqα (82% identical) and G11α (83% identical) from mouse. Sequences were aligned using the program Pileup (GCG). Dashes indicates amino acid identities, and dots indicate gaps. (B) Structure of the C. elegans Gqα gene, egl-30. The exons end in the middle or end of the codon for the following amino acids: exon 1, Gly (40); exon 2, Gln (82); exon 3, Tyr (155); exon 4, Arg (198); exon 5, Glu (241); exon 6, Gly (293); exon 7, Asp (329); exon 8, Val (355).
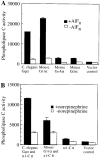
Figure 2. C. elegans Gqα Can Activate Phospholipase C
(A) C. elegans Gqα can stimulate endogenous phospholipase C in COS-7 cells. (B) C. elegans Gqα can be activated by an a adrenergic receptor. The data shown is the average of duplicate wells, and is representative of two other similar experiments. Units are cpm of [3H]inositol phosphates released. Assay of GoAα and Gi3α were performed in a separate experiment.
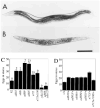
Figure 3. egl-30 Hermaphrodites Are Defective in Egg Laying
Wild-type (A) and egl-30(ad805) (B) animals 28 hr after L4 larval stage. Embryos that have started to undergo morphogenesis are visible inside egl-30 but not wild-type animals. Scale bar represents 0.2 mm. egl-30 homozygotes (C) and heterozygotes (D) retain eggs. At least 30 homozygotes and 7 heterozygotes of each allele were scored. The percentage of those eggs that were late stage (1 1/2 fold stage or later) is indicated above the bars in (C). No late stage eggs were found in (D). Homozygotes of egl-30(ad810) were not tested in this assay because of lethality and sterility. syEx125 in (C) is a multicopy extra-chromosomal array overexpressing the wild-type egl-30 gene. syEx125 suppresses the egg retention of egl-30(ad809), consistent with our identification of the Gqα gene as egl-30.
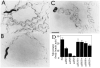
Figure 4. Mutations in egl-30 Disrupt Coordinated Movement
egl-30 mutations display reduced level of movement and leave flattened sinusoidal tracks. Single wild-type (A), egl-30(ad805) (B), or dpy-20(e1282); egl-30(syEx125) (C) adult hermaphrodites were placed on 2-day-old lawns of E. coli strain OP50, and allowed to swim for 1 hr. A portion of the tracks they left are shown. Scale bar represents 0.5 mm. (D) Movement level of selected egl-30 strains. Each bar represents the average of at least seven determinations.
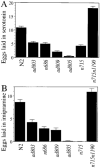
Figure 5. egl-30 Mutations Disrupt the Response of Egg-Laying Muscles to Serotonin and Imipramine
_egl-30_hermaphrodites lay a reduced number of eggs in serotonin (A) and imipramine (B). At least 30 unbloated animals were assayed for each drug and allele.
Similar articles
- Activation of EGL-47, a Galpha(o)-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior.
Moresco JJ, Koelle MR. Moresco JJ, et al. J Neurosci. 2004 Sep 29;24(39):8522-30. doi: 10.1523/JNEUROSCI.1915-04.2004. J Neurosci. 2004. PMID: 15456826 Free PMC article. - Participation of the protein Go in multiple aspects of behavior in C. elegans.
Mendel JE, Korswagen HC, Liu KS, Hajdu-Cronin YM, Simon MI, Plasterk RH, Sternberg PW. Mendel JE, et al. Science. 1995 Mar 17;267(5204):1652-5. doi: 10.1126/science.7886455. Science. 1995. PMID: 7886455 - Egg-laying.
Schafer WR. Schafer WR. WormBook. 2005 Dec 14:1-7. doi: 10.1895/wormbook.1.38.1. WormBook. 2005. PMID: 18050396 Free PMC article. Review. - The heterotrimeric G protein genes of Caenorhabditis elegans.
Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH. Jansen G, et al. Ernst Schering Res Found Workshop. 2000;(29):13-34. doi: 10.1007/978-3-662-04264-9_2. Ernst Schering Res Found Workshop. 2000. PMID: 10943302 Review. No abstract available.
Cited by
- Systematic profiling of Caenorhabditis elegans locomotive behaviors reveals additional components in G-protein Gαq signaling.
Yu H, Aleman-Meza B, Gharib S, Labocha MK, Cronin CJ, Sternberg PW, Zhong W. Yu H, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):11940-5. doi: 10.1073/pnas.1310468110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818641 Free PMC article. - Trio's Rho-specific GEF domain is the missing Galpha q effector in C. elegans.
Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, Tesmer JJ, Jorgensen EM, Wieland T, Miller KG. Williams SL, et al. Genes Dev. 2007 Nov 1;21(21):2731-46. doi: 10.1101/gad.1592007. Epub 2007 Oct 17. Genes Dev. 2007. PMID: 17942708 Free PMC article. - The G-protein gamma subunit gpc-1 of the nematode C.elegans is involved in taste adaptation.
Jansen G, Weinkove D, Plasterk RH. Jansen G, et al. EMBO J. 2002 Mar 1;21(5):986-94. doi: 10.1093/emboj/21.5.986. EMBO J. 2002. PMID: 11867526 Free PMC article. - Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans.
Govindan JA, Nadarajan S, Kim S, Starich TA, Greenstein D. Govindan JA, et al. Development. 2009 Jul;136(13):2211-21. doi: 10.1242/dev.034595. Development. 2009. PMID: 19502483 Free PMC article. - A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans.
Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG. Edwards SL, et al. PLoS Biol. 2008 Aug 5;6(8):e198. doi: 10.1371/journal.pbio.0060198. PLoS Biol. 2008. PMID: 18687026 Free PMC article.
References
- Avery L. Motor-neuron M3 controls pharyngeal muscle relaxation timing in Caenorhabditis elegans. J Exp Biol. 1993b;175:283–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL46154/HL/NHLBI NIH HHS/United States
- R01 HL046154/HL/NHLBI NIH HHS/United States
- F32NS09541-03/NS/NINDS NIH HHS/United States
- F32 NS009541/NS/NINDS NIH HHS/United States
- R37 HL046154/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases