A novel type of programmed neuronal death in the cervical spinal cord of the chick embryo - PubMed (original) (raw)
A novel type of programmed neuronal death in the cervical spinal cord of the chick embryo
H Yaginuma et al. J Neurosci. 1996.
Abstract
We examined the massive early cell death that occurs in the ventral horn of the cervical spinal cord of the chick embryo between embryonic days 4 and 5 (E4 and E5). Studies with immunohistochemical, in situ hybridization, and retrograde-tracing methods revealed that many dying cells express Islet proteins and Lim-3 mRNA (motoneuron markers) and send their axons to the somatic region of the embryo before cell death. Together, these data strongly suggest that the dying cells are somatic motoneurons. Cervical motoneurons die by apoptosis and can be rescued by treatment with cycloheximide and actinomycin D. Counts by motoneuron numbers between E3.5 and E10 revealed that, in addition to cell death between E4 and E5, motoneuron death also occur between E6 and E10 in the cervical cord. Studies with [3H]thymidine autoradiography and morphological techniques revealed that in the early cell-death phase (E4-E5), genesis of motoneurons, axonal elongation, and innervation of muscles is still ongoing. However, studies with [3H]thymidine autoradiography also revealed that the cells dying between E4 and E5 become postmitotic before E3.5. Increased size of peripheral targets, treatment with neuromuscular blockade, and treatment with partially purified muscle or brain extracts and defined neurotropic agents, such as NGF, BDNF, neurotrophin-3, CNTF, bFGF, PDGF, S100-beta, activin, cholinergic differentiation factor/leukemia inhibitory factor, bone morphogenetic protein-2, IGF-I, interleukin-6, and TGF-beta 1, were all ineffective in rescuing motoneurons dying between E4 and E5. By contrast, motoneurons that undergo programmed cell death at later stages (E6-E10) in the cervical cord are target-dependent and respond to activity blockade and trophic factors. Experimental approaches revealed that early cell death also occurs in a notochord-induced ectopic supernumerary motoneuron column in the cervical cord. Transplantation of the cervical neural tube to other segmental regions failed to alter the early death of motoneurons, whereas transplantation of other segments to the cervical region failed to induce early motoneuron death. These results suggest that the mechanisms that regulate motoneuron death in the cervical spinal cord between E4 and E5 are independent of interactions with targets. Rather, this novel type of cell death seems to be determined by signals that either are cell-autonomous or are derived from other cells within the cervical neural tube.
Figures
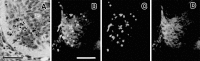
Fig. 1.
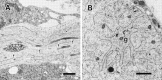
Photomicrographs of transverse sections through the cervical spinal cord of a st. 24 embryo showing the distribution of dying neurons in the ventral horn. A, Hematoxylin eosin staining. B–D, Double staining with the TUNEL method and SC1 (marker for ventral horn neurons) immunohistochemistry.B, Double staining; C, TUNEL; D, SC1. Scale bars, 50 μm. Arrows in A indicate pyknotic profiles of degenerating neurons.
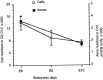
Fig. 2.
Line graphs showing numbers (mean ± SD) of pyknotic (dying and dead) cells in the ventral horn per 8-μm-thick section in the third, seventh, and eleventh cervical segments at st. 22 (E3.5) to st. 27 (E5.5). *C3 st. 24 vs C11 st. 24, p < 0.001; t test. Sample size = 4 at all stages.
Fig. 3.
Electron micrographs showing dying cells and other phagocytic cells that contain fragments (apoptotic bodies) of dead cells in the cervical ventral horn. A, Dying cells.B, Macrophage-like cell containing cell debris in the marginal zone. C, Cell debris contained in a neuroepithelial cell (arrow) in the marginal zone. Scale bars, 1 μm.
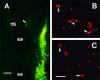
Fig. 4.
Top. A, A fluorescent, double-exposed photomicrograph showing cell nuclei (red) and Islet-1 immunopositive neurons in the ventral horn (green) at E4 (st. 23). Virtually all the cells in the ventral horn region express Islet-1 immunoreactivity. B, A fluorescent double-exposed photomicrograph taken by a confocal microscope showing colocalization of TUNEL positivity (red) and Islet-1 immunopositivity (green) in the nucleus of a dying neuron (arrow). Scale bars, 10 μm. The double-labeled cells are shown in yellow.
Fig. 6.
Photomicrographs showing neurons retrogradely labeled with FITC–latex beads. A, Fluorescent micrograph showing the injection site (i) and retrogradely labeled cells in the ipsilateral ventral horn. Dorsal toward the_top._ Scale bar, 100 μm. vh, Contralateral ventral horn; nc, notochord; ao, dorsal aorta.B, C, Fluorescent confocal micrographs showing colocalization of FITC–latex beads and TUNEL positivity. Scale bar in_C_, 10 μm for B, C. Arrows indicate FITC–latex beads located within TUNEL-positive profiles. Double labeling is shown in yellow.
Fig. 7.
Electron micrographs showing healthy and degenerating neurons retrogradely labeled by FITC–latex beads. The diameter of the beads shown here is 0.126 μm. To increase relative electron density of the beads, staining with uranyl acetate and lead citrate was omitted. A, The latex beads contained in a healthy cell. Note that the beads are surrounded by a cellular membrane (arrowheads). Scale bar, 0.5 μm. B, C, Latex beads observed in dying cells or in debris from dead cells (arrowheads). In C, the cell membrane around the latex bead can still be discerned. Scale bars, 1 μm.
Fig. 8.
The number (mean ± SD) of healthy cells in the ventral horn of lower cervical segments (C9–C12) at E6, E8, and E10, and axons in the ventral root of C10 at E6 and E10.
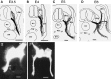
Fig. 9.
A–D, Camera lucida drawings showing the development of cervical spinal nerves from E3.5 to E6. dm: Somite (dermamyotome); drg: dorsal root ganglion;nc: notochord; sg: sympathetic ganglia;ao: aorta. Scale bars, 250 μm. E, F, Fluorescent photomicrographs of transverse sections through the cervical region of st. 21 (E) and st. 23 (F) embryos following DiI injection into the ventral region of the cervical cord. Dorsal is toward the top. Scale bars, 100 μm. Note that there is an apparent communicating branch (arrow in_E_) projecting from the spinal nerve toward the sympathetic ganglion. Virtually all such communicating branches between the spinal nerve and the sympathetic trunk disappear by st. 23 (F).
Fig. 10.
Axon numbers (mean ± SD) in the C10 ventral root between st. 21/22 (E3.5) and st. 28 (E5.5–6).
Fig. 11.
A, An electron micrograph of a transverse section through the ventral root of the cervical cord of a st. 25 embryo showing a degenerating axon (arrows). B, An electron micrograph of a section perpendicular to the C10 ventral root at st. 26. An apparent healthy-growth cone profile can be seen (g). Scale bars, 1 μm.
Fig. 12.
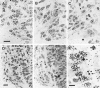
Micrographs showing transverse sections through the cervical–ventral horn of chick embryos treated with [3H]thymidine and processed for autoradiography. Dorsal is toward the top and medial is toward the right. A–C, Motoneurons that survived until E5.5. [3H]thymidine was administered at E3 (A), E3.5 (B), and E4 (C). Most of the neurons were labeled by [3H]thymidine administered at E3. The earliest developing unlabeled postmitotic motoneurons were located in the most ventromedial region of the ventral horn (arrows in_A_). Some motoneurons were labeled by [3H]thymidine administered at E4 (arrows in_C_). D, E, Dying and healthy neurons in the ventral horn region at E4.5. [3H]thymidine was administered at E3 (D) and E3.5 (E). Many cells in the ventral horn were labeled by [3H]thymidine administered at E3. After [3H]thymidine treatment at E3.5, most of the cells, including dying cells, in the lateral portion of ventral horn (lateral to the commissural fiber bundles) were not labeled. Labeled cells were located in the medial portion of the ventral horn (arrows). F, Higher magnification of_D_, showing labeled (arrowheads) and unlabeled (arrow) pyknotic profiles. Scale bar in A, 10 μm for A–C. Scale bar in D, 20 μm for_D, E._ Scale bar in F, 10 μm.
Fig. 13.
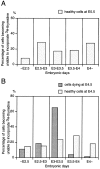
A, Bar graph showing birth dates of healthy cells in the ventral horn of the cervical cord at E5.5. B, Bar graph showing birth dates of those cells that die (are pyknotic) at E4.5, compared with the cells that are apparently healthy at E4.5 in the ventral horn. Abscissa indicates periods of development.Ordinate indicates percentage of cells that become unable to incorporate [3H]thymidine during each period, which was obtained by subtracting average percentage of cells unlabeled by [3H]thymidine treatment at the beginning of each period from that at the end of each period. Sample size = 2 for all stages. Note that most of the dying cells at E4.5 fail to incorporate [3H]thymidine (i.e., have become postmitotic) before E3.5, primarily between E3 and E3.5. Similar results were obtained with the use of another S-phase marker, bromodeoxyuridine (data not shown).
Fig. 14.
Pyknotic cells and axon numbers following curare treatment. A, Bar graph showing the numbers (mean ± SD) of pyknotic cells per 8-μm-thick section in the C10 segment at E4.5 following curare treatment. No significant differences were observed.B, Bar graph showing the number (mean ± SD) of axons in the C10 ventral root at E10 after continuous curare treatment from E3.5, E4.5, or E5.5 to E9.5. All experimental groups showed significant increases in axon numbers compared to controls (p < 0.001, ANOVA). However, no significant differences were observed_between_ experimental groups.
Fig. 15.
Bar graphs showing the results of transplantation of cervical segments to the cervical region (control) and to the brachial region. A, The number (mean ± SD) of pyknotic cells at E4.5 after experimental (cervical to brachial transplant) and control (cervical to cervical transplant) transplants was not significantly different. B, The number of Islet-1-immunopositive neurons in the ventral horn in 10-μm-thick section at E5 was not significantly different between control and experimental groups. C, Transplantation of the cervical neural tube to the brachial region resulted in approximately 30% more Islet-1-immunopositive neurons on E9. p < 0.001, t test.
Fig. 16.
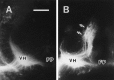
A, A fluorescent photomicrograph of a transverse section of an E7.5 chick embryo spinal cord showing the absence of a nucleus of Terni (sympathetic preganglionic neurons) in cervical segments transplanted into the thoracic region. B, A fluorescent photomicrograph of a transverse section of the E7.5 chick embryo spinal cord showing a nucleus of Terni (sympathetic preganglionic neurons) in thoracic segments transplanted into the cervical region. The nucleus of Terni (arrows) and motoneurons were retrogradely labeled by injection of DiI into the ventral root. Scale bar, 100 μm. VH, Ventral horn;FP, floor plate.
Fig. 17.
A, A line graph showing the difference in timing of cell death in the lower cervical cord (C11) between chick and quail. Sample size = 4 for both chick and quail. B, The number (mean ± SD) of pyknotic cells in cervical segments transplanted from chick to quail (experimental) and chick to chick (control). C, The number (mean ± SD) of pyknotic cells in cervical segments transplanted from quail to chick (experimental) and quail to quail (control). *p < 0.001, t test.
Fig. 18.
Fluorescent photomicrographs of transverse sections through the cervical (A) and brachial region (B) of E4.5 chick embryos that received a lateral notochord graft placed between the neural tube and somites on the right side at E1.5. Double labeling for SC1 (green) and TUNEL (red). In addition to the presence of normal ventral motoneurons [VH(L) and _VH(R)_], supernumerary motoneurons were induced laterally (VH′) by the implanted notochord. Cell death occurred in the supernumerary motoneuron column of the cervical spinal cord (A) but not in the brachial region (B). Scale bar, 50 μm.
Similar articles
- Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo.
Calderó J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Calderó J, et al. J Neurosci. 1998 Jan 1;18(1):356-70. doi: 10.1523/JNEUROSCI.18-01-00356.1998. J Neurosci. 1998. PMID: 9412513 Free PMC article. - Expression and regulation of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in distinct avian motoneuron subsets.
Kahane N, Shelton DL, Kalcheim C. Kahane N, et al. J Neurobiol. 1996 Mar;29(3):277-92. doi: 10.1002/(SICI)1097-4695(199603)29:3<277::AID-NEU1>3.0.CO;2-6. J Neurobiol. 1996. PMID: 8907158 - Neurotrophic interactions in the development of spinal cord motoneurons.
Oppenheim RW, Haverkamp LJ. Oppenheim RW, et al. Ciba Found Symp. 1988;138:152-71. doi: 10.1002/9780470513675.ch10. Ciba Found Symp. 1988. PMID: 3058426 Review. - Lower motoneuron trophic factors and lower motoneuron death: effects and mechanisms.
Raju TR, Bennett MR. Raju TR, et al. Aust Paediatr J. 1988;24 Suppl 1:48-9. Aust Paediatr J. 1988. PMID: 3060075 Review.
Cited by
- Programmed cell death of developing mammalian neurons after genetic deletion of caspases.
Oppenheim RW, Flavell RA, Vinsant S, Prevette D, Kuan CY, Rakic P. Oppenheim RW, et al. J Neurosci. 2001 Jul 1;21(13):4752-60. doi: 10.1523/JNEUROSCI.21-13-04752.2001. J Neurosci. 2001. PMID: 11425902 Free PMC article. - Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo.
Calderó J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Calderó J, et al. J Neurosci. 1998 Jan 1;18(1):356-70. doi: 10.1523/JNEUROSCI.18-01-00356.1998. J Neurosci. 1998. PMID: 9412513 Free PMC article. - Widespread elimination of naturally occurring neuronal death in Bax-deficient mice.
White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. White FA, et al. J Neurosci. 1998 Feb 15;18(4):1428-39. doi: 10.1523/JNEUROSCI.18-04-01428.1998. J Neurosci. 1998. PMID: 9454852 Free PMC article. - Programmed cell death in amyotrophic lateral sclerosis.
Guégan C, Przedborski S. Guégan C, et al. J Clin Invest. 2003 Jan;111(2):153-61. doi: 10.1172/JCI17610. J Clin Invest. 2003. PMID: 12531867 Free PMC article. Review. No abstract available. - Motor neurons and the generation of spinal motor neuron diversity.
Stifani N. Stifani N. Front Cell Neurosci. 2014 Oct 9;8:293. doi: 10.3389/fncel.2014.00293. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25346659 Free PMC article. Review.
References
- Abbott UK, Taylor LW, Abplanalp H. Studies with talpid, an embryonic lethal of the fowl. J Hered. 1960;51:195–202.
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
- Airey JA, Baring MD, Beck CF, Chelliah Y, Deerink TJ, Ellisman MH, Houenou LJ, McKenny DD, Sutko JL, Talvenheimo J. Failure to make normal alpha-ryanodine receptor is an early event associated with the crooked neck dwarf (cn ) mutation. Dev Dyn. 1993;197:169–188. - PubMed
- Bhattacharyya A, Oppenheim RW, Prevette D, Moore BW, Brackenbury R, Ratner N. S100 is present in developing chicken neurons and Schwann cells and promotes motor neuron survival in vivo. J Neurobiol. 1992;23:451–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources