Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody - PubMed (original) (raw)
Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody
P Dournaud et al. J Neurosci. 1996.
Abstract
Biological actions of somatostatin are exerted via a family of receptors, for which five genes recently have been cloned. However, none of these receptor proteins has been visualized yet in the brain. In the present-study, the regional and cellular distribution of the somatostatin sst2A receptor was investigated via immunocytochemistry in the rat central nervous system by using an antibody generated against a unique sequence of the receptor protein. Specificity of the antiserum was demonstrated by immunoblot and immunocytochemistry on rat brain membranes and/or on cells transfected with cDNA encoding the different sst receptor subtypes. In rat brain sections, sst2A receptor immunoreactivity was concentrated either in perikarya and dendrites or in axon terminals distributed throughout the neuropil. Somatodendritic labeling was most prominent in the olfactory tubercle, layers II-III of the cerebral cortex, nucleus accumbens, pyramidal cells of CA1-CA2 subfields of the hippocampus, central and cortical amygdaloid nuclei, and locus coeruleus. Labeled terminals were detected mainly in the endopiriform nucleus, deep layers of the cortex, claustrum, substantia innominata, subiculum, basolateral amygdala, medial habenula, and periaqueductal gray. Electron microscopy confirmed the association of sst2A receptors with perikarya and dendrites in the former regions and with axon terminals in the latter. These results provide the first characterization of the cellular distribution of a somatostatin receptor in mammalian brain. The widespread distribution of the sst2A receptor in cerebral cortex and limbic structures suggests that it is involved in the transduction of both pre- and postsynaptic effects of somatostatin on cognition, learning, and memory.
Figures
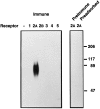
Fig. 1.
Western blot analysis of R2-88 immunoreactivity in CHO-K1 cells. Membranes (40 μg) from either parental CHO-K1 cells or from CHO-K1 cells transfected with cDNA encoding individual sst receptor subtypes were separated on SDS-PAGE. After transfer to PVDF membranes, the proteins were immunoblotted with a 1:10,000 dilution of R2-88 immune serum (Immune), R2-88 preimmune serum (PreImmune), or R2-88 immune serum in the presence of 1 μ
m
peptide antigen (Preadsorbed). Molecular size markers (in kDa) are shown on the_right_.
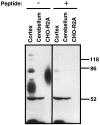
Fig. 2.
Western blot analysis of R2-88 immunoreactivity in rat brain. Membranes from rat cerebral cortex (50 μg), rat cerebellum (50 μg), or CHO-R2A cells (25 μg) were separated on a 10% SDS-acrylamide gel. After transfer to PVDF membranes, the proteins were immunoblotted with a 1:20,000 dilution of R2-88 immune serum in the absence or presence of 1 n
m
peptide antigen, as indicated. Molecular size markers (in kDa) are shown on the_right_.
Fig. 3.
Confocal imaging of COS-7 cells immunocytochemically stained for the sst2Areceptor. COS-7 cells transiently transfected with cDNA encoding the sst2A receptor exhibit intense cytoplasmic immunoreactivity (a). The yield of the transfection is ∼20%, which explains the presence of nonlabeled cells in the same field. No labeled cells are apparent in preparations incubated with preimmune serum (b) or with the R2-88 immune serum preadsorbed with an excess of peptide antigen (c). COS-7 cells transfected with cDNA encoding the sst2Breceptor are immunonegative, also (d). Magnification, 300×.
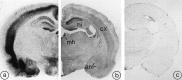
Fig. 4.
Comparative distribution of [125I]SRIF binding sites (a) and sst2A receptor immunoreactivity (b) in rat brain. Section in c was immunoreacted with R2-88 antiserum preadsorbed with an excess of the peptide antigen.a, Autoradiogram of a 20 μm rat midbrain section incubated with [125I]-Tyr0-DTrp8-SRIF-14 for 45 min. Reproduced from Moyse et al. (1992), with permission.b, As for [125I]SRIF binding, sst2A receptor immunoreactivity predominates in the deep layers of the cortex (cx), the medial habenula (mh), the hippocampal formation (hi), and the amygdaloid complex (am). c, By comparison, the preadsorbed control is virtually devoid of immunoreactivity. Magnification, 7×.
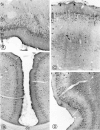
Fig. 5.
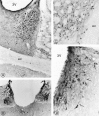
Light microscopic distribution of sst2A receptor immunoreactivity in cerebral cortex. A, Olfactory tubercle. Perikaryal immunostaining is prominent in cell bodies in the pyramidal (Py) and polymorph (Po) cell layers. Pl, Plexiform layer;Si, substantia innominata. Magnification, 250×.B, Anterior cingulate cortex. Numerous sst2A-immunoreactive perikarya are apparent throughout layer II. cc, Corpus callosum. Magnification, 170×. C, Parietal cortex. Immunoreactive nerve cell bodies are numerous in layers II–III and more sparsely distributed in layer V. Fields of labeled axon terminals are evident in deep layers, most prominently in layer V. Magnification, 270×. D, Dense perikaryal labeling is evident throughout layers II–III. Labeled terminal fields pervade the outer segment of layer V as well as layer VI. Magnification, 220×.
Fig. 6.
Distribution of sst2Areceptor immunoreactivity in the limbic system and neostriatum.A, Prominent perikaryal immunolabeling is detected throughout the lateral, intermediate, and ventral divisions of the bed nucleus of the stria terminalis. ic, Internal capsule;ac, anterior commissure; 3V, third ventricle. Magnification, 220×. B, Intense immunolabeling of nerve cell bodies and intervening neuropil pervades the medial habenular nucleus (Mh). Note the absence of labeling in the lateral division of the nucleus and the presence of immunoreactive axon terminals in the paraventricular nucleus of the thalamus (PV). Magnification, 270×. C, Small, intensely immunoreactive spiny type I neurons are evident in between the myelinated fascicles of the internal capsule in the ventrolateral neostriatum. ec, External capsule. Magnification, 420×.D, Dorsolateral septum. The labeling clearly is seen to pervade the cytoplasm of neuronal perikarya and their proximal dendrites (arrows). 3V, Third ventricle. Magnification, 850×.
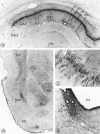
Fig. 7.
Distribution of sst2Areceptor immunoreactivity in the hippocampus, amygdala, and pons.A, Hippocampus. Intensely labeled pyramidal cells and proximal dendrites in subfields CA1–CA2 are superimposed over neuropil staining, stopping abruptly at CA3. or, Stratum oriens;py, stratum pyramidale; ra, stratum radiatum;lm, stratum lacunosum moleculare; Hil, hilus. Magnification, 140×. B, Temporal lobe. Intense immunoreactivity is evident in the central (Ce) and basolateral (Bla) amygdaloid nuclei. However, in the former, it is confined to nerve cell bodies and, in the latter, to axon terminals. Note the dense terminal labeling in the dorsal endopiriform nucleus (Den) and in the deep layers of the perirhinal cortex (CX). Cp, Caudate putamen; Pir, piriform cortex. Magnification, 100×. E, Labeled pyramidal cells in CA1. Apical dendrites are seen extending into the stratum radiatum (arrows). Note the sparing of the nucleus. Magnification, 1300×. D, Locus coeruleus (LC). Nerve cell bodies and surrounding neuropil are equally, densely immunoreactive. V4, 4th ventricle. Magnification, 100×.
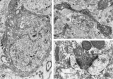
Fig. 8.
Electron microscopic detection of sst2A receptor protein in rat brain.A, Neuronal perikaryon in the medial habenula. The reaction product pervades the entire cytoplasm but spares the nucleus. Magnification, 8000×. B, Large varicose dendrite in the medial habenula. Both the upper and the lower dilatations are in synaptic contact (double arrows) with unlabeled terminals. Note the four neighboring cross-sectioned immunoreactive dendrites (d). One of these dendrites (*) is labeled less intensely than the others and is apposed directly to the large varicose one. Magnification, 11,500×. C, Labeled axon terminal (Lt) detected among unlabeled ones (Ut) in the basolateral amygdala. The labeled terminal forms asymmetric synaptic contacts (double arrows) with two immunonegative dendrites. Magnification, 32,000×.
Similar articles
- Interrelationships between somatostatin sst2A receptors and somatostatin-containing axons in rat brain: evidence for regulation of cell surface receptors by endogenous somatostatin.
Dournaud P, Boudin H, Schonbrunn A, Tannenbaum GS, Beaudet A. Dournaud P, et al. J Neurosci. 1998 Feb 1;18(3):1056-71. doi: 10.1523/JNEUROSCI.18-03-01056.1998. J Neurosci. 1998. PMID: 9437026 Free PMC article. - Immunohistochemical distribution of the somatostatin receptor subtype 5 in the adult rat brain: predominant expression in the basal forebrain.
Stroh T, Kreienkamp HJ, Beaudet A. Stroh T, et al. J Comp Neurol. 1999 Sep 13;412(1):69-82. doi: 10.1002/(sici)1096-9861(19990913)412:1<69::aid-cne5>3.0.co;2-v. J Comp Neurol. 1999. PMID: 10440710 - Plasticity of somatostatin and somatostatin sst2A receptors in the rat dentate gyrus during kindling epileptogenesis.
Csaba Z, Richichi C, Bernard V, Epelbaum J, Vezzani A, Dournaud P. Csaba Z, et al. Eur J Neurosci. 2004 May;19(9):2531-8. doi: 10.1111/j.0953-816X.2004.03361.x. Eur J Neurosci. 2004. PMID: 15128406 - Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies.
Schulz S, Händel M, Schreff M, Schmidt H, Höllt V. Schulz S, et al. J Physiol Paris. 2000 May-Aug;94(3-4):259-64. doi: 10.1016/s0928-4257(00)00212-6. J Physiol Paris. 2000. PMID: 11088003 Review. - Complementarity of radioautographic and immunohistochemical techniques for localizing neuroreceptors at the light and electron microscopy level.
Beaudet A, Dournaud P, Boudin H. Beaudet A, et al. Braz J Med Biol Res. 1998 Feb;31(2):215-23. doi: 10.1590/s0100-879x1998000200005. Braz J Med Biol Res. 1998. PMID: 9686144 Review.
Cited by
- Neurochemistry of the mammillary body.
Żakowski W, Zawistowski P. Żakowski W, et al. Brain Struct Funct. 2023 Jul;228(6):1379-1398. doi: 10.1007/s00429-023-02673-4. Epub 2023 Jun 28. Brain Struct Funct. 2023. PMID: 37378855 Free PMC article. Review. - A selective nonpeptide somatostatin receptor 5 agonist effectively decreases insulin secretion in hyperinsulinism.
Juliana CA, Chai J, Arroyo P, Rico-Bautista E, Betz SF, De León DD. Juliana CA, et al. J Biol Chem. 2023 Jun;299(6):104816. doi: 10.1016/j.jbc.2023.104816. Epub 2023 May 11. J Biol Chem. 2023. PMID: 37178920 Free PMC article. - Neuropeptide System Regulation of Prefrontal Cortex Circuitry: Implications for Neuropsychiatric Disorders.
Casello SM, Flores RJ, Yarur HE, Wang H, Awanyai M, Arenivar MA, Jaime-Lara RB, Bravo-Rivera H, Tejeda HA. Casello SM, et al. Front Neural Circuits. 2022 Jun 21;16:796443. doi: 10.3389/fncir.2022.796443. eCollection 2022. Front Neural Circuits. 2022. PMID: 35800635 Free PMC article. Review. - A simple novel approach for detecting blood-brain barrier permeability using GPCR internalization.
Csaba Z, Vitalis T, Charriaut-Marlangue C, Margaill I, Coqueran B, Leger PL, Parente I, Jacquens A, Titomanlio L, Constans C, Demene C, Santin MD, Lehericy S, Perrière N, Glacial F, Auvin S, Tanter M, Ghersi-Egea JF, Adle-Biassette H, Aubry JF, Gressens P, Dournaud P. Csaba Z, et al. Neuropathol Appl Neurobiol. 2021 Feb;47(2):297-315. doi: 10.1111/nan.12665. Epub 2020 Sep 27. Neuropathol Appl Neurobiol. 2021. PMID: 32898926 Free PMC article. - Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1.
Song YH, Hwang YS, Kim K, Lee HR, Kim JH, Maclachlan C, Dubois A, Jung MW, Petersen CCH, Knott G, Lee SH, Lee SH. Song YH, et al. Sci Adv. 2020 Apr 22;6(17):eaaz0517. doi: 10.1126/sciadv.aaz0517. eCollection 2020 Apr. Sci Adv. 2020. PMID: 32494634 Free PMC article.
References
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992;40:1457–1463. - PubMed
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law PY, Wessendorf MW, Elde R. The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA. 1995;92:5062–5066. - PMC - PubMed
- Beaudet A, Greenspun D, Raelson J, Tannenbaum GS. Patterns of expression of SSTR1 and SSTR2 somatostatin receptor subtypes in the hypothalamus of the adult rat: relationship to neuroendocrine function. Neuroscience. 1995;65:551–561. - PubMed
- Brown PJ, Lee AB, Norman MG, Presky DH, Schonbrunn A. Identification of somatostatin receptors by covalent labeling with a novel photoreactive somatostatin analog. J Biol Chem. 1990;265:17995–18004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous