The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis - PubMed (original) (raw)
The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis
M Ma et al. J Neurosci. 1996.
Abstract
The R20 neurons of Aplysia exhibit frequency-dependent spike broadening. Previously, we had used two-electrode voltage clamp to examine the mechanisms of this spike broadening (Ma and Koester, 1995). We identified three K+ currents that mediate action-potential repolarization: a transient A-type K+ current (I(Adepol)), a delayed rectifier current (IK-V), and a Ca(2+)-sensitive K+ current(IK-CA). A major constraint in that study was the lack of completely selective blockers for I(Adepol) and I(K-V), resulting in an inability to assess directly the effects of their activation and inactivation on spike broadening. In the present study, the dynamic-clamp technique, which employs computer simulation to inject biologically realistic currents into a cell under current-clamp conditions (Sharp et al., 1993a,b), was used either to block I(Adepol) or I(K-V) or to modify their inactivation properties. The data in this paper, together with earlier results, lead to the following hypothesis for the mechanism of spike broadening in the R20 cells. As the spike train progresses, the primary responsibility for spike repolarization gradually shifts from I(Adepol) to I(K-V) to I(K-Ca). This sequence can be explained on the basis of the relative rates of activation and inactivation of each current with respect to the constantly changing spike durations, the cumulative inactivation of I(Adepol) and I(K-V), and the progressive potentiation of I(K-Ca). Positive feedback interactions between spike broadening and inactivation contribute to the cumulative inactivation of both I(Adepol) and I(K-V). The data also illustrate that when two or more currents have similar driving forces and partially overlapping activation characteristics, selectively blocking one current under current-clamp conditions can lead to a significant underestimate of its normal physiological importance.
Figures
Fig. 3.
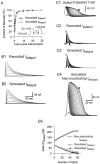
A mathematical model, which accurately simulates I_Adepol, illustrates the role of cumulative inactivation in the evolution of_I_Adepol traces during a spike train.A, The recovery from inactivation of recorded_I_Adepol has two time constants. A two-pulse protocol (inset) was used to determine the recovery from inactivation caused by the first 100 msec pulse from −50 to +30 mV. The peak current during the second pulse, normalized to that during the first one (filled circles), was plotted against the time interval between the two pulses. The curve (smooth line) was fit by a double exponential function; the major component had a τ_h value of 0.9 sec, whereas τ_h_ for the minor component was 18.5 sec. To simulate _I_Adepol, the recovery from inactivation was approximated by using just the faster time constant, which was 1.1 sec at −50 mV, averaged from four experiments. The accuracy of this approximation was tested by using the same double-pulse protocol (inset) to drive the dynamic-clamp circuit in open-loop mode. The peak current calculated during the second pulse, normalized to that during the first pulse (open circle), was plotted against the time interval between two pulses, which was fit by a single exponential curve (dotted line). B, The model simulates _I_Adepol during rectangular depolarizing steps. B1, Empirically measured_I_Adepol in response to 200 msec depolarizing steps in 10 mV increments from −40 to +30 mV from a holding potential of −50 mV. B2, _I_Adepol was simulated by driving the dynamic-clamp circuit in open-loop mode with the same set of depolarizing steps as those used in B1. C, The simulated _I_Adepol traces during action-potential trains were quite similar to those recorded as 4-AP difference current. C1, An action-potential train evoked by injecting brief, depolarizing current pulses into the soma was recorded for use as a command signal. The holding potential was −40 mV.C2, _I_Adepol was recorded as a 4-AP difference current under voltage clamp while playing back the spike train recorded in C1. C3, C4,_I_Adepol was simulated by playing back the action-potential train from C1 to the dynamic-clamp circuit in open-loop mode with two different versions of the model. C3,I_Adepol waveforms that were simulated with the unmodified version of the model matched the empirically determined values in C2. C4, When noninactivating_I_Adepol was simulated by fixing_h = 1, the same command led to greatly enhanced values of _I_Adepol. C5, Changes in peak values of recorded and simulated I_Adepol from_C2–C4 are plotted against spike number._I_Adepol was measured as a 1 m
m
4-AP difference current after 60 μ
m
TTX, 2 m
m
CdCl2, and 40 m
m
TEA had been added to block_I_Na, _I_K-Ca, and_I_K-V. The value of G_maxused in the simulations C3, C4 was 1900 nS. The first and last traces in C are indicated by the_white and black arrows, respectively.
Fig. 1.
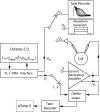
The dynamic clamp was used in open-loop mode to simulate current waveforms off-line in response to predetermined voltage commands consisting of either (1) a train of recorded spike waveforms, (2) rectangular voltage steps, or (3) in closed-loop mode to inject, block, or modify currents on-line.
Fig. 10.
Addition of either _I_Adepolor _I_K-V with the dynamic clamp was sufficient to cause spike broadening in a cell in which K+ currents had been preblocked pharmacologically. An action-potential train was evoked: A, In normal ASW. B, After 50 m
m
TEA and 10 m
m
4-AP had been added to block_I_K-V, _I_K-Ca, and_I_Adepol. C, With both_I_Adepol and _I_K-V added back. D, With _I_Adepol added back.E, With _I_K-V added back.F, With _I_K-V, modified to express only the slow, nonstate-dependent inactivation, added back. Resting potential was −47 mV. _G_max for_I_Adepol, 1500 nS; _G_maxfor _I_K-V, 1500 nS (n = 6).
Fig. 2.
Characteristic patterns of changes in individual currents accompany frequency-dependent spike broadening. A 7 Hz, 9.3 sec action-potential train (left, first row) was evoked by injecting brief, depolarizing current pulses into a cell with a resting potential of −48 mV. The five major ionic currents mediating the spike train were isolated by playing back the spike train as the command to the conventional voltage clamp. Between each repetition of the train, the following sequence of blocking drugs was added, one at a time, to the bath: 60 μ
m
TTX, 3 m
m
TEA, 40 m
m
TEA, 1 m
m
4-AP, or 2 m
m
CdCl2. The resulting difference currents measured before and after adding each compound revealed _I_Na,_I_K-Ca, _I_K-V,_I_Adepol, and _I_Ca, respectively. The five major currents during the first spike, the 27th spike (at which point _I_K-V reached its peak), the 43rd spike (at which point the maximum broadening was reached), and the last spike (65th) are shown. The normalized values of spike duration and of the time integrals of each current are plotted against spike number in the right-hand column.
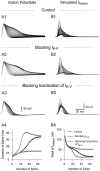
Fig. 4.
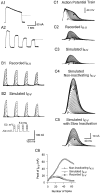
A mathematical model, which accurately simulates_I_K-V, illustrates the role of cumulative inactivation in the evolution of _I_K-V traces during a spike train. A, _I_K-Vexhibits state-dependent inactivation, which causes it to inactivate more rapidly in response to several brief pulses than to one long pulse of equivalent duration. Depolarizing steps were from a holding potential of −50 mV to +20 mV. A1,I_K-V inactivated relatively slowly during a 2 sec depolarizing step. A2, I_K-Vduring four 500 msec pulses repeated at 1 Hz decayed to a lower final level than in A1. The dotted line in_A1 marks the corresponding level of_I_K-V at the end of four brief voltage steps in_A2. B, Recorded and simulated_I_K-V were similar during 50 msec repetitive depolarizing pulses (−10 to +50 mV) at 7 Hz from a holding potential of −50 mV. B1, _I_K-V was measured empirically as a TEA difference current using the voltage-step protocol shown in the inset. B2, _I_K-V was simulated by driving the dynamic-clamp circuit in open-loop mode.C, The simulated waveforms of _I_K-Vduring action-potential trains were similar to those recorded as TEA difference currents. C1, An action-potential train evoked by injecting brief depolarizing current pulses was recorded for use as a command signal. The holding potential was −50 mV. C2,_I_K-V was recorded as a TEA difference current under voltage clamp while playing back the spike train recorded in_C1._C3–C5, _I_K-V was simulated by playing back the action-potential train from C1 to the dynamic-clamp circuit in open-loop mode with three different versions of the model.C3, Simulated I_K-V had normal inactivation. C4, Noninactivating_I_K-V was simulated by fixing h = 1. C5, The state-dependent nature of_I_K-V inactivation was ignored; τ_h was estimated from the decay during long (2 sec) voltage steps (A1) rather than from high-frequency trains of steps as in B. C6, Peak values of recorded and simulated I_K-V from_C3–C5 are plotted against spike number._I_K-V was measured as a 40 m
m
TEA difference current after 60 m
m
TTX and 2 m
m
CdCl2 had been added to block _I_Na,_I_Ca, and _I_K-Ca. The value of _G_max used for the simulations in _C_was 1850 nS. The first and last traces in C are indicated by the white and black arrows, respectively.
Fig. 5.
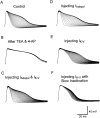
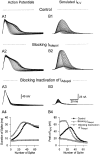
Blocking _I_Adepol activation (or inactivation) with the dynamic clamp accelerated (or eliminated) spike broadening, which in turn modified the dynamics of_I_K-V activation and inactivation. A, Action-potential trains were evoked under control conditions (A1), after blocking _I_Adepol(A2), or after blocking inactivation of_I_Adepol (A3). The dynamic clamp was used in closed-loop mode either to simply cancel out_I_Adepol (A2) or to replace it with a noninactivating version of _I_Adepol(A3). B, Simulated _I_K-Vchanged during a spike train recorded while_I_Adepol (or its inactivation) was blocked.B1, Simulated _I_K-V during a control spike train showed bimodal changes. B2, Simulated_I_K-V during an action-potential train recorded while _I_Adepol was blocked underwent cumulative inactivation from the increased initial value. B3, Simulated_I_K-V during a spike train recorded while inactivation of _I_Adepol was blocked underwent only modest cumulative inactivation. A4, B4, Changes in spike width (A1–A3) and peak values of simulated_I_K-V (B1–B3) caused by modifying_I_Adepol are plotted against spike number. Resting potential was −50 mV. _G_max used by the dynamic clamp for blocking or modifying _I_Adepolwas 1650 nS. _G_max used for simulating_I_K-V in B1–B3 was 2050 nS.
Fig. 6.
Blocking _I_Adepol activation with the dynamic clamp accelerated spike broadening, which, in turn, enhanced the activation of _I_K-Ca and modified its kinetics. A, The spike trains recorded either under control conditions (A1) or with_I_Adepol activation blocked (A2) by the dynamic clamp were used as commands to the voltage clamp for isolating _I_K-Ca pharmacologically (B1, B2). B, _I_K-Ca was measured as 3 m
m
TEA difference currents under control condition (B1) and after blocking _I_Adepol(B2). A3, B3, The durations of the spikes of the two trains (A1, A2) and the corresponding peaks of_I_K-Ca currents (B1, B2) are plotted against spike number. Resting potential was −50 mV, and_G_max used for the _I_Adepolsimulations (A2) was 1350 nS.
Fig. 9.
Using the dynamic clamp to block activation or inactivation of _I_Adepol or of_I_K-V caused changes in frequency-dependent spike broadening that were measured in the same cell. A, Action-potential trains generated with K+ conductances in various different states. A1, Control; A2, with_I_Adepol blocked; A3, with_I_K-V blocked; A4, with_I_Adepol and _I_K-V blocked;A5, with inactivation of _I_Adepolblocked; A6, with inactivation of_I_K-V blocked. B, Durations of spikes in A1–A6 are plotted against spike number. Resting potential was −50 mV. Currents were blocked or modified by using_G_max for _I_Adepol = 1750 nS and _G_max for _I_K-V = 1650 nS.
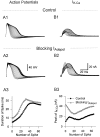
Fig. 7.
Blocking _I_K-V activation (or inactivation) with the dynamic clamp accelerated (or reduced) spike broadening, which, in turn, modified the rate and extent of_I_Adepol inactivation. A, Action-potential trains were evoked under control conditions (A1), after blocking _I_K-V(A2), or after blocking inactivation of_I_K-V (A3). The dynamic clamp was used in closed-loop mode either to simply cancel out_I_K-V (A2) or to replace it with a noninactivating version of _I_K-V (A3).B, Compared with control (B1), simulated_I_Adepol underwent faster inactivation during a spike train with _I_K-V blocked (B2) or slower and less complete inactivation with inactivation of_I_K-V blocked (B3). A4, B4, Changes in spike width (A1–A3) and peak values of_I_Adepol (B1–B3) caused by modifying_I_K-V are plotted against spike number. Resting potential was −50 mV. _G_max used by the dynamic clamp for blocking or modifying _I_K-V was 1700 nS. _G_max of _I_Adepol used for simulating _I_Adepol in B1–B3 was 1950 nS.
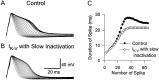
Fig. 8.
Replacing the rapid, state-dependent inactivation of _I_K-V by a slower, Hodgkin–Huxley-type version reduced both the rate and final extent of spike broadening.A, An action-potential train was evoked by injecting brief depolarizing current pulses under control conditions. B, An action-potential train was evoked after eliminating the state-dependent inactivation of _I_K-V, which was achieved by injecting two currents into the cell simultaneously:_I_K-V with normal state-dependent inactivation but with reversed sign to block the existing_I_K-V of the cell and _I_K-Vwith only slow inactivation and normal polarity to replace the endogenous current. C, The durations of spikes for these two trains are plotted against the number of spike. Resting potential was −48 mV. _G_max used by the dynamic clamp for blocking or modifying _I_K-V was 2100 nS.
Fig. 11.
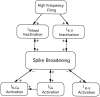
Summary of the predominant mechanisms underlying spike broadening in the R20 neurons, determined in this and the previous study (Ma and Koester, 1995). Some interactions that play a minor role in spike broadening are not included in this diagram. For example, spike broadening also facilitates the activation of_I_Adepol (Fig. 3_C4_). This effect is overwhelmed by the progressive increase in inactivation, however. Broadening also enhances cumulative inactivation of_I_Ca (Ma and Koester, 1995). This effect has two opposing effects on spike broadening: by reducing inward current it limits broadening, and by reducing the build-up of_I_K-Ca, it enhances broadening.
Similar articles
- Consequences and mechanisms of spike broadening of R20 cells in Aplysia californica.
Ma M, Koester J. Ma M, et al. J Neurosci. 1995 Oct;15(10):6720-34. doi: 10.1523/JNEUROSCI.15-10-06720.1995. J Neurosci. 1995. PMID: 7472431 Free PMC article. - Activators of protein kinase C mimic serotonin-induced modulation of a voltage-dependent potassium current in pleural sensory neurons of Aplysia.
Sugita S, Baxter DA, Byrne JH. Sugita S, et al. J Neurophysiol. 1994 Sep;72(3):1240-9. doi: 10.1152/jn.1994.72.3.1240. J Neurophysiol. 1994. PMID: 7807208 - Computational model of the serotonergic modulation of sensory neurons in Aplysia.
Baxter DA, Canavier CC, Clark JW Jr, Byrne JH. Baxter DA, et al. J Neurophysiol. 1999 Dec;82(6):2914-35. doi: 10.1152/jn.1999.82.6.2914. J Neurophysiol. 1999. PMID: 10601429 - Differential effects of 4-aminopyridine, serotonin, and phorbol esters on facilitation of sensorimotor connections in Aplysia.
Sugita S, Baxter DA, Byrne JH. Sugita S, et al. J Neurophysiol. 1997 Jan;77(1):177-85. doi: 10.1152/jn.1997.77.1.177. J Neurophysiol. 1997. PMID: 9120559 - Different mechanisms underlying the repolarization of narrow and wide action potentials in pyramidal cells and interneurons of cat motor cortex.
Chen W, Zhang JJ, Hu GY, Wu CP. Chen W, et al. Neuroscience. 1996 Jul;73(1):57-68. doi: 10.1016/0306-4522(96)00010-3. Neuroscience. 1996. PMID: 8783229
Cited by
- Ionic mechanisms of endogenous bursting in CA3 hippocampal pyramidal neurons: a model study.
Xu J, Clancy CE. Xu J, et al. PLoS One. 2008 Apr 30;3(4):e2056. doi: 10.1371/journal.pone.0002056. PLoS One. 2008. PMID: 18446231 Free PMC article. - Characteristics of single large-conductance Ca2+-activated K+ channels and their regulation of action potentials and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus.
Lin M, Hatcher JT, Wurster RD, Chen QH, Cheng ZJ. Lin M, et al. Am J Physiol Cell Physiol. 2014 Jan 15;306(2):C152-66. doi: 10.1152/ajpcell.00423.2012. Epub 2013 Nov 6. Am J Physiol Cell Physiol. 2014. PMID: 24196530 Free PMC article. - Voltage-Gated Potassium Channels Ensure Action Potential Shape Fidelity in Distal Axons.
Gonzalez Sabater V, Rigby M, Burrone J. Gonzalez Sabater V, et al. J Neurosci. 2021 Jun 23;41(25):5372-5385. doi: 10.1523/JNEUROSCI.2765-20.2021. Epub 2021 May 17. J Neurosci. 2021. PMID: 34001627 Free PMC article. - Slow conductances could underlie intrinsic phase-maintaining properties of isolated lobster (Panulirus interruptus) pyloric neurons.
Hooper SL, Buchman E, Weaver AL, Thuma JB, Hobbs KH. Hooper SL, et al. J Neurosci. 2009 Feb 11;29(6):1834-45. doi: 10.1523/JNEUROSCI.5392-08.2009. J Neurosci. 2009. PMID: 19211890 Free PMC article. - Maternal diabetes increases large conductance Ca2+-activated K+ outward currents that alter action potential properties but do not contribute to attenuated excitability of parasympathetic cardiac motoneurons in the nucleus ambiguus of neonatal mice.
Lin M, Hatcher JT, Chen QH, Wurster RD, Li L, Cheng ZJ. Lin M, et al. Am J Physiol Regul Integr Comp Physiol. 2011 May;300(5):R1070-8. doi: 10.1152/ajpregu.00470.2010. Epub 2011 Jan 19. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21248308 Free PMC article.
References
- Baukrowitz T, Yellen G. Modulation of K+current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous