Increased expression of IL-1beta converting enzyme in hippocampus after ischemia: selective localization in microglia - PubMed (original) (raw)
Increased expression of IL-1beta converting enzyme in hippocampus after ischemia: selective localization in microglia
R V Bhat et al. J Neurosci. 1996.
Abstract
Although the interleukin-1beta converting enzyme (ICE)/CED-3 family of proteases has been implicated recently in neuronal cell death in vitro and in ovo, the role of specific genes belonging to this family in cell death in the nervous system remains unknown. To address this question, we examined the in vivo expression of one of these genes, Ice, after global forebrain ischemia in gerbils. Using RT-PCR and Western immunoblot techniques, we detected an increase in the mRNA and protein expression of ICE in hippocampus during a period of 4 d after ischemia. Chromatin condensation was observed in CA1 neurons within 2 d after ischemia. Internucleosomal DNA fragmentation and apoptotic bodies were observed between 3 and 4 d after ischemia, a period during which CA1 neuronal death is maximal. In nonischemic brains, ICE-like immunoreactivity was relatively low in CA1 pyramidal neurons but high in scattered hippocampal interneurons. After ischemia, ICE-like immunoreactivity was not altered in these neurons. ICE-like immunoreactivity, however, was observed in microglial cells in the regions adjacent to the CA1 layer as early as 2 d after ischemic insult. The increase in ICE-like immunoreactivity was robust at 4 d after ischemia, a period that correlates with the DNA fragmentation observed in hippocampal homogenates of ischemic brains. These results provide the first evidence for the localization and induction of ICE expression in vivo after ischemia and suggest an indirect role for ICE in ischemic damage through mediation of an inflammatory response.
Figures
Fig. 1.
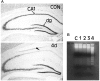
A, Loss of CA1 pyramidal neurons 4 d after global forebrain ischemia. Sections from nonischemic control (CON) gerbil hippocampus and hippocampus obtained from gerbils 4 d (4d) after ischemia were stained with cresyl violet. Low-power photomicrographs show the cell loss in the CA1 region of the hippocampus (arrowhead) 4 d after global forebrain ischemia. dg, Dentate gyrus. B, Time course of DNA fragmentation in hippocampal tissue after ischemia. Genomic DNA was extracted from ischemic and nonischemic hippocampi and subjected to gel electrophoresis. Laddering of fragmented DNA is observed between 3 and 4 d after ischemia.
Fig. 2.
Morphological evidence for a- poptosis in CA1 neurons after ischemia. A, Normal CA1 pyramidal neurons from nonischemic gerbil. B, Margination of condensed chromatin along the nuclear envelope of CA1 neurons 2 d after ischemia. Some neurons exhibit nuclear shrinkage (inset). Occasionally microglia are seen attached to a neuronal cell body (arrowhead). C, Most CA1 neurons have shrunken nuclei by 3 d after ischemia. Clumping of chromatin and late stages of apoptotic morphology are evident. Numerous microglia are found in the CA1 region. D, End-stage apoptosis is evident by 4 d after ischemia (arrow). Some neurons exhibit morphological features of necrosis (inset).
Fig. 3.
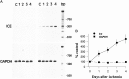
RT-PCR analysis of the time course of_Ice_ mRNA after ischemia. A, The time course of expression of Ice mRNA levels in sham-operated and in ischemic gerbil hippocampi shows that low levels of Ice mRNA are detected in sham-operated and nonischemic gerbil hippocampus. After ischemia, an increase in Ice mRNA levels is observed as early as 24 hr and continues to increase during the 4 d after ischemia. mRNA isolated from the same tissues was subjected to RT-PCR using primers for the GAPDH gene. No significant alteration in mRNA expression is observed for this gene. B, Representation of amplified products (volumetric quantitation) as a percentage of nonischemic control at various post-ischemic intervals.
Fig. 4.
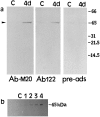
Western immunoblot analysis of ICE expression.a, Twenty micrograms of protein from hippocampal homogenates from control (C) and 4 d post-ischemic brains were subjected to gel electrophoresis, and the blots were probed with polyclonal antibodies to ICE (AbM-20 and Ab122). Both antibodies detect an increase in the 45 kDa immunoreactive species (arrowhead) 4 d after ischemia. Preadsorption with 100-fold excess peptide prepared against Ab122 significantly reduced the intensity of this band. b, Time course of ICE protein expression detected by ABM-20 in hippocampal homogenates in nonischemic control (C) and 1–4 d after ischemia.
Fig. 5.
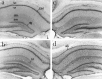
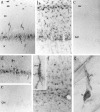
Increased expression of ICE-like immunoreactivity in hippocampus after ischemia. Gerbils were perfusion-fixed and 50 μm frozen sections were stained free-floating for ICE immunoreactivity using AbM-20. Low-power photomicrographs show (a) ICE-like immunoreactivity in control (nonischemic) brain section (b) 2 d after ischemia, (c) 3 d after ischemia, and (d) 4 d after ischemia. so, Stratum oriens;sr, stratum radiatum; slm, stratum lacunosum-moleculare; sm, stratum moleculare; hf, hippocampal fissure; alv, alveus; dg, dentate gyrus.
Fig. 6.
Immunolocalization of ICE in microglia after ischemia. a, High-power photomicrograph of ICE-like immunoreactivity localized to interneurons (arrowheads) in the CA1 region of the hippocampus of a nonischemic brain using AbM-20.I, Interneurons; so, stratum oriens;sr, stratum radiatum; alv, alveus. b, High-power photomicrograph of ICE-like immunoreactivity in the CA1 region of the hippocampus of a 4 d ischemic brain showing an increase in ICE-like immunoreactivity in microglia. alv, Alveus. c, Lack of immunostaining in the absence of primary antibody. d, A second antibody (Ab122) showing a similar pattern of immunostaining in nonischemic CA1 sector of hippocampus.I, interneurons. e, Reduction in staining with the Ab-122 in the presence of 100-fold excess blocking peptide in the nonischemic CA1 sector of hippocampus. f, Increased ICE-like immunoreactivity in the region of the hippocampal fissure (hf) 4 d after ischemia. High-magnification photomicrograph showing ICE-like immunoreactivity in microglia (m, inset). sr, Stratum radiatum;slm, stratum lacunosum-moleculare. g, High-magnification photomicrograph showing a composite of an ICE-positive microglial cell (m) in the process of attaching to an ICE-positive interneuron (I).
Similar articles
- Global ischemia induces apoptosis-associated genes in hippocampus.
Honkaniemi J, Massa SM, Breckinridge M, Sharp FR. Honkaniemi J, et al. Brain Res Mol Brain Res. 1996 Nov;42(1):79-88. doi: 10.1016/s0169-328x(96)00121-0. Brain Res Mol Brain Res. 1996. PMID: 8915583 - Activation of CPP-32 protease in hippocampal neurons following ischemia and epilepsy.
Gillardon F, Böttiger B, Schmitz B, Zimmermann M, Hossmann KA. Gillardon F, et al. Brain Res Mol Brain Res. 1997 Oct 15;50(1-2):16-22. doi: 10.1016/s0169-328x(97)00162-9. Brain Res Mol Brain Res. 1997. PMID: 9406913 - Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis.
Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, Kominami E, Uchiyama Y. Nitatori T, et al. J Neurosci. 1995 Feb;15(2):1001-11. doi: 10.1523/JNEUROSCI.15-02-01001.1995. J Neurosci. 1995. PMID: 7869078 Free PMC article. - Interneurons in rat hippocampus after cerebral ischemia. Morphometric, functional, and therapeutic investigations.
Johansen FF. Johansen FF. Acta Neurol Scand Suppl. 1993;150:1-32. Acta Neurol Scand Suppl. 1993. PMID: 7907456 Review. - Regulation of ICE activity and ICE isoforms by LPS.
Tingsborg S, Ziólkowska M, Zetterström M, Hasanvan H, Bartfai T. Tingsborg S, et al. Mol Psychiatry. 1997 Mar;2(2):122-4. doi: 10.1038/sj.mp.4000224. Mol Psychiatry. 1997. PMID: 9106233 Review.
Cited by
- Caspase-1 affects chronic restraint stress-induced depression-like behaviors by modifying GABAergic dysfunction in the hippocampus.
Li M, Sun X, Wang Z, Li Y. Li M, et al. Transl Psychiatry. 2023 Jun 27;13(1):229. doi: 10.1038/s41398-023-02527-x. Transl Psychiatry. 2023. PMID: 37369673 Free PMC article. - Is longer sevoflurane preconditioning neuroprotective in permanent focal cerebral ischemia?
Qiu C, Sheng B, Wang S, Liu J. Qiu C, et al. Neural Regen Res. 2013 Aug 15;8(23):2126-33. doi: 10.3969/j.issn.1673-5374.2013.23.002. Neural Regen Res. 2013. PMID: 25206521 Free PMC article. - Disruption of the serotonergic system after neonatal hypoxia-ischemia in a rodent model.
Buller KM, Wixey JA, Reinebrant HE. Buller KM, et al. Neurol Res Int. 2012;2012:650382. doi: 10.1155/2012/650382. Epub 2012 Feb 8. Neurol Res Int. 2012. PMID: 22474587 Free PMC article. - Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats.
Mawhinney LJ, de Rivero Vaccari JP, Dale GA, Keane RW, Bramlett HM. Mawhinney LJ, et al. BMC Neurosci. 2011 Dec 1;12:123. doi: 10.1186/1471-2202-12-123. BMC Neurosci. 2011. PMID: 22133203 Free PMC article. - Cornel iridoid glycoside inhibits inflammation and apoptosis in brains of rats with focal cerebral ischemia.
Ya BL, Li CY, Zhang L, Wang W, Li L. Ya BL, et al. Neurochem Res. 2010 May;35(5):773-81. doi: 10.1007/s11064-010-0134-2. Epub 2010 Feb 13. Neurochem Res. 2010. PMID: 20155318
References
- Ayala JM, Yamin T-T, Egger LA, Chin J, Kostura MJ, Miller DK. IL-1β-converting enzyme is present in monocytic cells as an inactive 45 kDa precursor. J Immunol. 1994;153:2592–2599. - PubMed
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Ness KV, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, Huebner K, Black RA. Molecular cloning of the interleukin-1β converting enzyme. Science. 1992;256:97–100. - PubMed
- Clarke PGH. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. - PubMed
- Colton CA, Gilbert DL. Production of the superoxide anion by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous