Cerebral representation of one's own past: neural networks involved in autobiographical memory - PubMed (original) (raw)
Cerebral representation of one's own past: neural networks involved in autobiographical memory
G R Fink et al. J Neurosci. 1996.
Abstract
We studied the functional anatomy of affect-laden autobiographical memory in normal volunteers. Using H2 15O positron emission tomography (PET), we measured changes in relative regional cerebral blood flow (rCBF). Four rCBF measurements were obtained during three conditions: REST, i.e.,, subjects lay at rest (for control); IMPERSONAL, i.e., subjects listened to sentences containing episodic information taken from an autobiography of a person they did not know, but which had been presented to them before PET scanning (nonautobiographical episodic memory ecphory); and PERSONAL, i.e., subjects listened to sentences containing information taken from their own past (autobiographical episodic memory ecphory). Comparing IMPERSONAL with REST (nonautobiographical episodic memory ecphory) resulted in relative rCBF increases symmetrically in both temporal lobes including the temporal poles and medial and superior temporal gyri. The same loci, however, with a stronger lateralization to the right hemisphere were activated in the comparison PERSONAL to REST (autobiographical episodic memory ecphory). In addition, the right temporomesial, right dorsal prefrontal, right posterior cingulate areas, and the left cerebellum were activated. A comparison of PERSONAL and IMPERSONAL (autobiographical vs nonautobiographical episodic memory ecphory) demonstrated a preponderantly right hemispheric activation including primarily right temporomesial and temporolateral cortex, right posterior cingulate areas, right insula, and right prefrontal areas. The right temporomesial activation included hippocampus, parahippocampus, and amygdala. These results suggest that a right hemispheric network of temporal, together with posterior, cingulate, and prefrontal, areas is engaged in the ecphory of affect-laden autobiographical information.
Figures
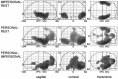
Fig. 1.
Relative rCBF increases during nonautobiographical and autobiographical episodic memory retrieval. Relative rCBF increases for the group of seven subjects associated with nonautobiographical episodic memory retrieval (IMPERSONAL–REST), autobiographical episodic memory retrieval (PERSONAL–REST), and autobiographical memory (PERSONAL–IMPERSONAL). Areas of significant (p < 0.05, corrected for multiple nonindependent comparisons) relative rCBF increases associated with the different tasks are shown as through projections onto representations of the standard stereotactic space as defined by Talairach and Tournoux (1988). The sagittal images (left) view the brain from the side, the coronal images (middle) view the brain from the back, and the transverse images (right) view the brain from the top. R, Right; VAC, vertical plane through the anterior commissure; VPC, vertical plane through the posterior commissure. Numbers at axes refer to coordinates of stereotactic space. The exact coordinates of the local maxima within areas of activation and their Z statistics are given in Table 1.
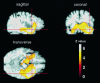
Fig. 2.
Functional anatomy of temporal activations during affect-laden autobiographical memory. Same data as in Figure 1, but here the group SPM{t} map has been sectioned in sagittal, coronal, and transverse planes and is displayed on top of an arbitrary MR image that has been normalized spatially to the same anatomical space. The red cross-hair indicates the local maximum within the area of activation. The _color bar_indicates the Z statistics achieved (Z value). This figure details the functional anatomy of temporal activations associated with autobiographical memory (PERSONAL–IMPERSONAL) and their relationship to underlying anatomy. Note that the activations are predominantly on the right (left image corresponds to subjects’s left) and include temporomedial, temporolateral, and insular areas.
Similar articles
- Differential modulation of a common memory retrieval network revealed by positron emission tomography.
Maguire EA, Mummery CJ. Maguire EA, et al. Hippocampus. 1999;9(1):54-61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. Hippocampus. 1999. PMID: 10088900 - Neural correlates of semantic and episodic memory retrieval.
Wiggs CL, Weisberg J, Martin A. Wiggs CL, et al. Neuropsychologia. 1999 Jan;37(1):103-18. doi: 10.1016/s0028-3932(98)00044-x. Neuropsychologia. 1999. PMID: 9920476 Clinical Trial. - Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories.
Markowitsch HJ, Vandekerckhove MM, Lanfermann H, Russ MO. Markowitsch HJ, et al. Cortex. 2003 Sep-Dec;39(4-5):643-65. doi: 10.1016/s0010-9452(08)70858-x. Cortex. 2003. PMID: 14584547 Clinical Trial. - Recollections of one's own past: the effects of aging and gender on the neural mechanisms of episodic autobiographical memory.
Piefke M, Fink GR. Piefke M, et al. Anat Embryol (Berl). 2005 Dec;210(5-6):497-512. doi: 10.1007/s00429-005-0038-0. Anat Embryol (Berl). 2005. PMID: 16172875 Review. - Mapping episodic memory.
Nyberg L. Nyberg L. Behav Brain Res. 1998 Feb;90(2):107-14. doi: 10.1016/s0166-4328(97)00094-6. Behav Brain Res. 1998. PMID: 9521543 Review.
Cited by
- Determining functional connectivity using fMRI data with diffusion-based anatomical weighting.
Bowman FD, Zhang L, Derado G, Chen S. Bowman FD, et al. Neuroimage. 2012 Sep;62(3):1769-79. doi: 10.1016/j.neuroimage.2012.05.032. Epub 2012 May 24. Neuroimage. 2012. PMID: 22634220 Free PMC article. - Behavioral and neuroanatomical investigation of Highly Superior Autobiographical Memory (HSAM).
LePort AK, Mattfeld AT, Dickinson-Anson H, Fallon JH, Stark CE, Kruggel F, Cahill L, McGaugh JL. LePort AK, et al. Neurobiol Learn Mem. 2012 Jul;98(1):78-92. doi: 10.1016/j.nlm.2012.05.002. Epub 2012 May 29. Neurobiol Learn Mem. 2012. PMID: 22652113 Free PMC article. - Task-related, low-frequency task-residual, and resting state activity in the default mode network brain regions.
Zhang S, Li CS. Zhang S, et al. Front Psychol. 2012 May 30;3:172. doi: 10.3389/fpsyg.2012.00172. eCollection 2012. Front Psychol. 2012. PMID: 22661964 Free PMC article. - The cerebellum plays a role in conscious episodic memory retrieval.
Andreasen NC, O'Leary DS, Paradiso S, Cizadlo T, Arndt S, Watkins GL, Ponto LL, Hichwa RD. Andreasen NC, et al. Hum Brain Mapp. 1999;8(4):226-34. doi: 10.1002/(SICI)1097-0193(1999)8:4<226::AID-HBM6>3.0.CO;2-4. Hum Brain Mapp. 1999. PMID: 10619416 Free PMC article. - An rTMS study into self-face recognition using video-morphing technique.
Heinisch C, Dinse HR, Tegenthoff M, Juckel G, Brüne M. Heinisch C, et al. Soc Cogn Affect Neurosci. 2011 Sep;6(4):442-9. doi: 10.1093/scan/nsq062. Epub 2010 Jun 29. Soc Cogn Affect Neurosci. 2011. PMID: 20587597 Free PMC article.
References
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. - PubMed
- Barbas H. Anatomical basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. - PubMed
- Barker WW, Yoshii F, Loewenstein DA, Chang JY, Apicella A, Pascal S, Boothe TE, Ginsberg MD, Duara R. Cerebrocerebellar relationship during behavioral activation: a PET study. J Cereb Blood Flow Metab. 1991;11:48–54. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous