A distinct pattern of trophic factor expression in myelin-deficient nerves of Trembler mice: implications for trophic support by Schwann cells - PubMed (original) (raw)
A distinct pattern of trophic factor expression in myelin-deficient nerves of Trembler mice: implications for trophic support by Schwann cells
H C Friedman et al. J Neurosci. 1996.
Abstract
Distal to a peripheral nerve transection, myelin degradation and Schwann cell (SC) proliferation are accompanied by a marked upregulation of brain-derived neurotrophic factor (BDNF) and a decrease of ciliary neurotrophic factor (CNTF) in non-neuronal cells. To investigate the role of SC differentiation in trophic factor regulation, we studied BDNF and CNTF expression in sciatic nerves from Trembler-J (Tr-J) mice. In these animals, a mutation in the pmp-22 gene causes a failure of myelination and continuous SC proliferation, but axonal continuity is preserved. In spite of the severe abnormalities in Tr-J nerves, BDNF levels remained as low as in the intact controls. Thus, the primary SC disorder in Tr-J produces a different pattern of BDNF expression from that caused by axonal breakdown due to nerve transection. Furthermore, the upregulation of BDNF mRNA triggered by transection was 70-fold in control nerves, but only 30-fold in Tr-J sciatic nerves. Because these results raised the possibility that axonal loss may influence neurotrophin expression only in SCs that have differentiated toward a myelinating phenotype, we measured BDNF mRNA after axotomy in the cervical sympathetic trunk (CST), a predominantly unmyelinated autonomic nerve. In contrast to the sciatic nerves, the BDNF mRNA level barely increased in the injured CST, supporting the idea that not all SCs are equal sources of trophic molecules. In Tr-J sciatic nerves, CNTF mRNA levels were fourfold lower than normal, implying that the downregulation of this cytokine is a sensitive indicator of a spectrum of SC perturbations that affect myelinating cells.
Figures
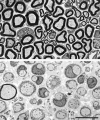
Fig. 1.
Electron micrographs of transverse sections from normal (top) and Tr-J(bottom) ventral roots. In the Tr-J_nerves, most axons are ensheathed by SC cytoplasm without myelin. A few axons are thinly myelinated. There are more SC nuclei in the_Tr-J nerve than in the control nerve. Scale bar, 10 μm.
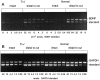
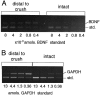
Fig. 2.
RT-PCR of BDNF and GAPDH mRNAs in_Tr-J_ and normal sciatic nerves. RNA was purified from distal stumps of transected sciatic nerves 2 weeks after axotomy. Intact contralateral nerves were used as controls. After RT with random primers, the cDNA samples were amplified for BDNF (A) and GAPDH (B). Each sample was coamplified with a dilution series of five concentrations of the BDNF DNA standard. For example, in the intact Tr-J nerves, the intensity of the BDNF cDNA band (318 bp) is greater than the 8 × 10−4amol standard band (197 bp) and less than the 4 × 10−4 amol band, giving a measurement of 6–7 × 10−4 amol for this sample. By the same procedure, the GAPDH mRNA of this sample was estimated to be 2.8 amol (B), giving a ratio of 0.02% BDNF/GAPDH.
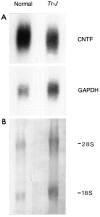
Fig. 3.
Northern blot of CNTF and GAPDH RNAs in normal and Tr-J sciatic nerves. Total RNA from 3–5 sciatic nerves of control and Tr-J mice was processed for Northern blot hybridization as described in Materials and Methods (A). The blot was first hybridized with a CNTF cDNA probe and subsequently rehybridized with a GAPDH cDNA probe. In the_Tr-J_ nerves, the GAPDH mRNA was proportional to the main rRNA species (18S and 28S), visualized when the blot was stained with methylene blue (B). S, Svedberg units.
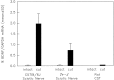
Fig. 4.
Effect of transection on BDNF/GAPDH ratios in sciatic nerves from control (C57Bl/6J) and_Tr-J_ mice and in rat CSTs. BDNF and GAPDH mRNAs were quantitated using competitive RT-PCR (as in Fig. 2) of RNA purified from the distal segments of transected and uninjured nerves. After transection (black bars), the mean ± SD of BDNF/GAPDH ratios are increased for both the normal (p = 0.02) and Tr-J nerves (p = 0.04; Mann–Whitney rank sum test). In addition, the increase in BDNF/GAPDH ratio was significantly less for the Tr-J nerves than the controls (p = 0.01; Student’s _t_test). BDNF mRNA was not detectable in the RNA prepared from the intact rat CSTs; 2 weeks after transection, the BDNF/GAPDH ratio was substantially less in the CST than in the sciatic nerve preparations.
Fig. 5.
RT-PCR of BDNF and GAPDH mRNAs in CSTs after nerve crush. RNA was purified from four intact and four distal stumps of crushed rat CSTs 1 week after axotomy. The cDNA pool produced by random-primed RT was amplified in PCR for BDNF (A) and GAPDH (B). The GAPDH measurements for intact and crushed CSTs were 0.4 and 2.2 amol. The BDNF level of four intact CSTs was less than or equal to the detection limit of 0.0004 amol; crushed CSTs measured 0.0016 amol BDNF. The average BDNF/GAPDH ratio of two experiments with crushed CSTs was 0.09 ± 0.01%. std., Standard.
Similar articles
- Regulation of ciliary neurotrophic factor expression in myelin-related Schwann cells in vivo.
Friedman B, Scherer SS, Rudge JS, Helgren M, Morrisey D, McClain J, Wang DY, Wiegand SJ, Furth ME, Lindsay RM, et al. Friedman B, et al. Neuron. 1992 Aug;9(2):295-305. doi: 10.1016/0896-6273(92)90168-d. Neuron. 1992. PMID: 1497895 - Ciliary neurotrophic factor expression in Schwann cells is induced by axonal contact.
Lee DA, Zurawel RH, Windebank AJ. Lee DA, et al. J Neurochem. 1995 Aug;65(2):564-8. doi: 10.1046/j.1471-4159.1995.65020564.x. J Neurochem. 1995. PMID: 7616210 - Altered slow axonal transport and regeneration in a myelin-deficient mutant mouse: the trembler as an in vivo model for Schwann cell-axon interactions.
de Waegh S, Brady ST. de Waegh S, et al. J Neurosci. 1990 Jun;10(6):1855-65. doi: 10.1523/JNEUROSCI.10-06-01855.1990. J Neurosci. 1990. PMID: 2355253 Free PMC article. - Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival.
Sendtner M, Arakawa Y, Stöckli KA, Kreutzberg GW, Thoenen H. Sendtner M, et al. J Cell Sci Suppl. 1991;15:103-9. doi: 10.1242/jcs.1991.supplement_15.14. J Cell Sci Suppl. 1991. PMID: 1824101 Review. - Regulation of myelin-specific gene expression. Relevance to CMT1.
Kamholz J, Awatramani R, Menichella D, Jiang H, Xu W, Shy M. Kamholz J, et al. Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
Cited by
- Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment.
Pramatarova A, Laganière J, Roussel J, Brisebois K, Rouleau GA. Pramatarova A, et al. J Neurosci. 2001 May 15;21(10):3369-74. doi: 10.1523/JNEUROSCI.21-10-03369.2001. J Neurosci. 2001. PMID: 11331366 Free PMC article. - Abnormal Schwann cell/axon interactions in the Trembler-J mouse.
Robertson AM, King RH, Muddle JR, Thomas PK. Robertson AM, et al. J Anat. 1997 Apr;190 ( Pt 3)(Pt 3):423-32. doi: 10.1046/j.1469-7580.1997.19030423.x. J Anat. 1997. PMID: 9147228 Free PMC article. - Concepts for regulation of axon integrity by enwrapping glia.
Beirowski B. Beirowski B. Front Cell Neurosci. 2013 Dec 19;7:256. doi: 10.3389/fncel.2013.00256. Front Cell Neurosci. 2013. PMID: 24391540 Free PMC article. Review. - Rapid axoglial signaling mediated by neuregulin and neurotrophic factors.
Esper RM, Loeb JA. Esper RM, et al. J Neurosci. 2004 Jul 7;24(27):6218-27. doi: 10.1523/JNEUROSCI.1692-04.2004. J Neurosci. 2004. PMID: 15240814 Free PMC article. - New evidence for secondary axonal degeneration in demyelinating neuropathies.
Moss KR, Bopp TS, Johnson AE, Höke A. Moss KR, et al. Neurosci Lett. 2021 Jan 23;744:135595. doi: 10.1016/j.neulet.2020.135595. Epub 2020 Dec 24. Neurosci Lett. 2021. PMID: 33359733 Free PMC article. Review.
References
- Acheson A, Barker PA, Alderson RF, Miller FD, Murphy RA. Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron. 1991;7:265–275. - PubMed
- Aguayo AJ, Epps J, Charron L, Bray GM. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves. Quantitative microscopy and radioautography. Brain Res. 1976;104:1–20. - PubMed
- Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas-Pérez MP, Vidal-Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond Biol. 1991;331:337–343. - PubMed
- Ayers MM, Anderson RM. Onion bulb neuropathy in the Trembler mouse: a model of hypertrophic interstitial neuropathy (Dejerine-Sottas) in man. Acta Neuropathol. 1973;25:54–70. - PubMed
- Bradley WG, Asbury AK. Duration of synthesis phase in neurilemma cells in mouse sciatic nerve during degeneration. Exp Neurol. 1970;26:275–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases