Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer's disease - PubMed (original) (raw)
Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer's disease
R E Mrak et al. J Neuropathol Exp Neurol. 1996 Mar.
Abstract
The neurite extension factor S100 beta is overexpressed by activated astrocytes associated with amyloid-containing plaques in Alzheimer's disease, and has been implicated in dystrophic neurite formation in these plaques. This predicts (a) that the appearance of S100beta- immunoreactive (S100beta+) astrocytes precedes that of dystrophic neurites in diffuse amyloid deposits and (b) that the number of these astrocytes correlates with the degree of dystrophic neurite proliferation in neuritic plaques. As a test of the first prediction, we determined the number of S100beta+ astrocytes associated with different plaque types: diffuse non-neuritic, diffuse neuritic, dense-core neuritic, and dense-core non-neuritic. Diffuse non-neuritic plaques had small numbers of associated S100beta+ astrocytes (1.3 +/- 0.1 S100beta astrocytes per plaque [mean +/- SEM]; 80% of plaques had one or more). These astrocytes were most abundant in diffuse neuritic plaques (4.2 +/- 0.2; 100%), were somewhat less numerous in dense-core neuritic plaques (1.6 +/- 0.2; 90%), and were only rarely associated with dense-core non-neuritic plaques (0.15 +/- 0.05; 12%). As a test of the second prediction, we correlated the number of S100beta+ astrocytes per plaque with the area of beta-amyloid precursor protein (beta-APP) immunoreactivity per plaque (an index of the size of the plaques' dystrophic neurite shells) and found a significant positive correlation (r = 0.74, p < 0.001). This correlation was also evident at the tissue level: the numbers of S100beta+ astrocytes per plaque-rich field correlated with the total area beta-APP immunoreactivity in these fields (r = 0.66, p < 0.05). These correlations support the idea that astrocytic activation and S100 beta overexpression are involved in the induction and maintenance of dystrophic neurites in amyloid deposits, and support the concept of a glial cytokine-mediated cascade underlying the progression of neuropathological changes in Alzheimer's disease.
Figures
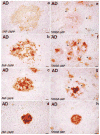
Fig. 1
Examples of plaque types dual-immunolabeled for β-amyloid (β-AP, brown) and β-amyloid precursor protein (β-APP, red) (left column, a–d) or for S100β (S100β, brown) and β-amyloid (β-AP, red) (right column, e–h). (a, e) Diffuse non-neuritic plaques devoid of condensed amyloid and β-APP+ neurites, and with several associated S100β+ activated astrocytes, (b, f) Diffuse neuritic plaques with both diffuse and condensed amyloid as well as dystrophic β-APP+ neurites, but without a dense β-amyloid core, and with an abundance of associated activated S100β+ astrocytes, (c, g) Dense-core neuritic plaques with compact round core deposits, halos of diffuse amyloid, β-APP+ neurites, and several associated S100β+ activated astrocytes, (d, h) Dense-core, non-neuritic plaques devoid of diffuse amyloid, β-APP+ neurites, and S100β+ astrocytes. Arrowheads denote examples of S100β+ astrocytes. Bars = 15 μm.
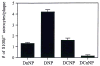
Fig. 2
Number of S100β+ astrocytes associated with each of the 4 defined plaque types. DnNP = diffuse non-neuritic plaques; DNP = diffuse neuritic plaques; DCNP = dense-core neuritic plaques; DCnNP = dense-core, non-neuritic plaques. Data expressed as mean ± SEM for 60 plaques of each type (5 in each of 12 patients). In each case, the number of S100β+ astrocytes associated with plaque types was significantly different from that of the postulated predecessor plaque type (i.e. the plaque type to the left in the figure); p < 0.001 in each case.
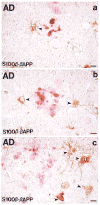
Fig. 3
Photomicrograph of S100β/β-APP dual-immunolabeled tissue sections showing β-amyloid precursor protein-immunoreactive dystrophic neurites (red) and associated S100β immunoreactive astrocytes (brown; arrowheads denote examples of S100β+ astrocytes) in neuritic plaques of 3 sizes. Note the greater number of S100β+ astrocytes associated with the larger plaques (b and c compared with a). Bars = 15 μm.
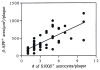
Fig. 4
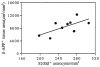
Positive correlation between β-APP+ dystrophic neurite cross-sectional area and the number of associated S100β+ astrocytes for 50 neuritic plaques from 7 Alzheimer patients (r = 0.74; p = 0.001).
Fig. 5
Positive correlation between β-APP+ dystrophic neurite cross sectional area and the number of S100β+ astrocytes within plaque-rich fields in adjacent sections of parahippocampal cortex from each of 9 Alzheimer patients (r = 0.86; p < 0.05).
Similar articles
- Overexpression of the neuritotrophic cytokine S100beta precedes the appearance of neuritic beta-amyloid plaques in APPV717F mice.
Sheng JG, Mrak RE, Bales KR, Cordell B, Paul SM, Jones RA, Woodward S, Zhou XQ, McGinness JM, Griffin WS. Sheng JG, et al. J Neurochem. 2000 Jan;74(1):295-301. doi: 10.1046/j.1471-4159.2000.0740295.x. J Neurochem. 2000. PMID: 10617132 Free PMC article. - [Expression of cytokine IL-1α and S100β in different types of plaques in Alzheimer's disease].
Yao JJ, He SR, Chen L, Yang L, Qiao XB, Zhang W, Du J, Liu DG. Yao JJ, et al. Zhonghua Bing Li Xue Za Zhi. 2011 Sep;40(9):581-4. Zhonghua Bing Li Xue Za Zhi. 2011. PMID: 22177239 Chinese. - Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution.
Griffin WS, Sheng JG, Roberts GW, Mrak RE. Griffin WS, et al. J Neuropathol Exp Neurol. 1995 Mar;54(2):276-81. doi: 10.1097/00005072-199503000-00014. J Neuropathol Exp Neurol. 1995. PMID: 7876895 - Neuritic Plaques - Gateways to Understanding Alzheimer's Disease.
Tsering W, Prokop S. Tsering W, et al. Mol Neurobiol. 2024 May;61(5):2808-2821. doi: 10.1007/s12035-023-03736-7. Epub 2023 Nov 8. Mol Neurobiol. 2024. PMID: 37940777 Free PMC article. Review. - The role of activated astrocytes and of the neurotrophic cytokine S100B in the pathogenesis of Alzheimer's disease.
Mrak RE, Griffinbc WS. Mrak RE, et al. Neurobiol Aging. 2001 Nov-Dec;22(6):915-22. doi: 10.1016/s0197-4580(01)00293-7. Neurobiol Aging. 2001. PMID: 11754999 Review.
Cited by
- Blood biomarkers for brain injury: What are we measuring?
Kawata K, Liu CY, Merkel SF, Ramirez SH, Tierney RT, Langford D. Kawata K, et al. Neurosci Biobehav Rev. 2016 Sep;68:460-473. doi: 10.1016/j.neubiorev.2016.05.009. Epub 2016 May 12. Neurosci Biobehav Rev. 2016. PMID: 27181909 Free PMC article. Review. - Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer's disease.
Matsuoka Y, Picciano M, Malester B, LaFrancois J, Zehr C, Daeschner JM, Olschowka JA, Fonseca MI, O'Banion MK, Tenner AJ, Lemere CA, Duff K. Matsuoka Y, et al. Am J Pathol. 2001 Apr;158(4):1345-54. doi: 10.1016/S0002-9440(10)64085-0. Am J Pathol. 2001. PMID: 11290552 Free PMC article. - Effects of S100B on Serotonergic Plasticity and Neuroinflammation in the Hippocampus in Down Syndrome and Alzheimer's Disease: Studies in an S100B Overexpressing Mouse Model.
Shapiro LA, Bialowas-McGoey LA, Whitaker-Azmitia PM. Shapiro LA, et al. Cardiovasc Psychiatry Neurol. 2010;2010:153657. doi: 10.1155/2010/153657. Epub 2010 Aug 22. Cardiovasc Psychiatry Neurol. 2010. PMID: 20827311 Free PMC article. - Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer's pathology.
Rangasamy SB, Jana M, Roy A, Corbett GT, Kundu M, Chandra S, Mondal S, Dasarathi S, Mufson EJ, Mishra RK, Luan CH, Bennett DA, Pahan K. Rangasamy SB, et al. J Clin Invest. 2018 Oct 1;128(10):4297-4312. doi: 10.1172/JCI96209. Epub 2018 Jul 10. J Clin Invest. 2018. PMID: 29990310 Free PMC article. - Brain structure and cognitive correlates of body mass index in healthy older adults.
Bolzenius JD, Laidlaw DH, Cabeen RP, Conturo TE, McMichael AR, Lane EM, Heaps JM, Salminen LE, Baker LM, Scott SE, Cooley SA, Gunstad J, Paul RH. Bolzenius JD, et al. Behav Brain Res. 2015 Feb 1;278:342-7. doi: 10.1016/j.bbr.2014.10.010. Epub 2014 Oct 22. Behav Brain Res. 2015. PMID: 25448431 Free PMC article.
References
- Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. - PubMed
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. - PubMed
- Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL. A4 protein in Alzheimer’s disease: Primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol. 1989;48:674–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials