Rapid, active hair bundle movements in hair cells from the bullfrog's sacculus - PubMed (original) (raw)
Rapid, active hair bundle movements in hair cells from the bullfrog's sacculus
M E Benser et al. J Neurosci. 1996.
Abstract
Hair bundles, the mechanically sensitive organelles of hair cells in the auditory and vestibular systems, are elastic structures that are deflected by sound or acceleration. To examine rapid mechanical events associated with mechanoelectrical transduction, we stimulated individual hair bundles with flexible glass fibers and measured their responses with a temporal resolution of 400 microsec. When a hair bundle from the bullfrog's sacculus was abruptly deflected in the positive direction, the bundle's motion in the direction of stimulation was interrupted within the initial few milliseconds by an active movement, or twitch. This response was biphasic, with an initial component in the direction of the stimulus and a second component in the opposite direction. The amplitude and duration of the twitch depended on the bundle's initial position and the size and rise time of the stimulus; the twitch was largest over the range of bundle deflections in which transduction was most sensitive. Under displacement clamp conditions, in which a hair bundle's position was changed and then held constant with negative feedback, the twitch manifested itself as a biphasic force exerted by the bundle. Some hair bundles produced twitches in response to negatively directed stimuli, exhibited stimulus-evoked damped oscillations, or twitched spontaneously. The hair bundle's ability to perform work against an external load and to oscillate in response to stimulation indicates that the bundle could supply feedback for mechanical amplification in vertebrate auditory organs.
Figures
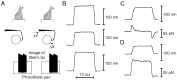
Fig. 1.
The experimental system and control experiments.A, In preparation for stimulation, the stimulus fiber’s tip was brought into contact with the bundle at its kinociliary bulb, and the displacement monitor was centered over the fiber’s tip. After application of a displacement to the fiber’s base, the bundle’s resultant movement was measured by the displacement monitor. The displacement monitor reported a voltage that was proportional to the difference in light received by its two photocells. B, In a base movement experiment, the base of a stimulus fiber of stiffness 1190 μN · m−1 was abruptly displaced (bottom trace). When the fiber’s tip was unengaged, it followed the displacement pulse with high fidelity (top trace). When the same fiber’s tip was attached to a flexible glass filament of stiffness 511 μN · m−1, the tip’s movement (middle trace) was diminished as a result of the stimulus fiber’s flexion. C, Under displacement clamp conditions, the tip displacement of an unloaded stimulus fiber of stiffness 595 μN · m−1 (top trace) essentially reached its final value within 2 msec. The force measured by the clamp system (bottom trace) reflected the viscous drag on the fiber, whose drag coefficient was 198 nN · sec · m−1. D, When the same fiber was attached to a flexible glass filament of stiffness 820 μN · m−1, the tip motion (top trace) was equally fast. The clamp force (bottom trace) showed both a steady-state component attributable to the elastic load and transients arising from viscous drag. The data for panels_B_, C, and D were filtered at 10 kHz and sampled at 25 kHz; each trace is the average of 10 responses.
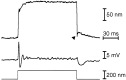
Fig. 10.
Displacement clamp measurement of the twitch force. A, When a pulse displacement command (top trace) was applied to the clamp system, the fiber’s base underwent a convoluted excursion (second trace) while effecting the commanded hair bundle displacement (third trace). The total force exerted by the fiber ( fourth trace) was determined by multiplication of its flexion, the difference between the second and third traces, by its calibrated stiffness of 951 μN · m−1. The hydrodynamic damping force on the fiber and bundle, which produced transients at the onset and conclusion of the displacement pulse, was calculated as the product of the fiber tip’s instantaneous velocity and the summed drag coefficients of the fiber and bundle (Howard and Hudspeth, 1988). Subtraction of this force component yielded the force exerted by the bundle (fifth trace), which was directed opposite to that produced by the fiber. The downward deflections_at the left of three traces (arrows) demonstrate the displacement clamp’s effectiveness. When the photodiode pair in the displacement monitor was subjected to a 20 μm calibration pulse, the clamp circuit produced a compensatory, 20 nm movement of the fiber’s tip. The record of bundle motion consequently shows almost no signal, while the records of fiber base displacement and the force traces display the response. These records indicate that the clamp system was, in this instance, 95% effective at controlling movement of the fiber’s tip. B, C, Comparison of the same hair bundle’s responses under base movement conditions (top traces) and displacement clamp conditions (bottom traces) demonstrates the relation between the twitch and the associated bundle forces on two time scales. The positively directed initial component of the twitch is associated with a positively directed force exerted by the bundle (left vertical line in C). The twitch’s second, negatively directed component corresponds to a negatively directed bundle force (right vertical line in C). The fiber’s damping constant was 79.5 nN · sec · m−1, and its base displacement for the_top traces of B and C was 120 nm. The traces in A represent the averages of five results; those in B and C are the averages of 10 results.
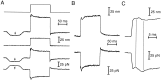
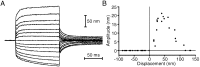
Fig. 2.
The hair bundle’s evoked mechanical twitch.A, A hair bundle was stimulated by application of a 175 nm displacement pulse (bottom trace) at the base of a flexible fiber whose tip was coupled to the bundle’s top. After displacement of the bundle toward its tall edge, a positive stimulus, the bundle’s movement (top trace) in the direction of the applied force was briefly interrupted by a biphasic twitch (arrowhead). A spike of depolarizing receptor potential (middle trace) coincided with the twitch.B, When the same hair bundle was subjected to a negative displacement pulse of an identical magnitude, a twitch and transient depolarization occurred during the positively directed bundle motion at the end of the stimulus (arrowhead). C, A temporally expanded presentation of the bundle motion in_A_ demonstrates the measurement of a twitch’s size. The amplitude of a twitch was taken as the displacement between its peak and nadir, as shown by the coarse horizontal lines. Back-extrapolation of the subsequent bundle motion ( fine line) yielded an alternative, larger estimate of a twitch’s size. The upper time calibration applies only to_C_. All traces are averages of 10 records. The fiber’s stiffness was 293 μN · m−1; the cell’s resting potential was −63 mV.
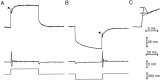
Fig. 3.
Control experiments. A, A twitch occurred after stimulation of a hair bundle in the positive direction (top trace). When the same bundle was stimulated in an orthogonal direction, however, the twitch was absent (middle trace). The response returned when the direction of stimulation was restored to the bundle’s plane of symmetry (bottom trace). The slow bundle relaxation in the middle panel likely reflected a stimulus that was not perfectly perpendicular to the bundle’s axis of responsiveness. B, A twitch occurred in the saline solution used for most experiments (top trace). Substitution of saline solution containing 100 μ
m
gentamicin suppressed the twitch (middle trace), which returned after restoration of the original solution (bottom trace). After exposure to aminoglycoside drugs, both evoked and spontaneous twitching were potentiated for several minutes (J. Howard, personal communication).C, An intact hair bundle produced a twitch (top trace). This response was not materially affected by detaching the kinocilium from the hair bundle and immobilizing it against the apical cellular surface with a microelectrode (bottom trace). D, A hair bundle bathed in standard saline solution containing 4 m
m
Ca2+ produced a twitch (top trace). After replacement of this solution with one containing 250 μ
m
Ca2+, the same bundle produced a twitch of similar magnitude but greater duration (bottom trace). In the presence of a Ca2+concentration similar to that of endolymph, mechanical stimulation often produced oscillatory bundle movements at a frequency (here 65 Hz) similar to that at which saccular afferent fibers are tuned (Koyama et al., 1982). Stimulus fiber base displacements for A,B, C, and D were 130, 140, 300, and 143 nm, respectively; the fiber stiffnesses were 448, 353, 137, and 535 μN · m−1, respectively; the traces represent the averages of 12, 14–16, 10, and 10 responses, respectively.
Fig. 4.
A twitch evoked by a negatively directed stimulus component. Driven by a 213 nm movement of the fiber’s base (bottom trace), the hair bundle’s movement (top trace) included both a positively directed twitch at the pulse’s outset and a negatively directed twitch (arrowhead) at the pulse’s conclusion. Unlike the initial twitch, the mechanical response at the pulse’s end was not associated with a strong spike of depolarizing receptor potential (middle trace). The results from 10 stimuli were averaged. The fiber’s stiffness was 283 μN · m−1; the cell’s resting potential was −56 mV.
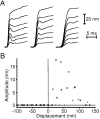
Fig. 5.
The twitch’s dependence on hair bundle displacement, as determined by application of variable-amplitude displacement pulses to the base of a stimulus fiber. A, This bundle’s superimposed responses showed that the twitch grew in amplitude with increasing bundle displacement for values up to ∼40 nm; for greater bundle displacements, the twitch’s amplitude progressively declined to zero. B, The twitch’s amplitude is plotted as a function of hair bundle displacement. In_A_, 14 step displacements, each 400 msec in duration, were delivered to the fiber’s base; the steps were uniformly spaced between and included −400 and 400 nm. An additional record was obtained in the absence of a displacement. The results from 10 stimulus wave trains were averaged; averaged responses of <1 nm, which did not significantly exceed the noise level, are plotted as zero. The data in_B_ were obtained from the same hair bundle by three repetitions of the procedure used to obtain A. The fiber’s stiffness was 293 μN · m−1.
Fig. 6.
Effect of stimulus rise time on the amplitude and time course of twitches. A, Sets of variable-amplitude stimuli were delivered to a hair bundle, with each set low-pass-filtered at a different frequency. The twitch’s duration increased and its amplitude declined with increasing rise time. The half-power frequencies of stimulus filtering were 500 Hz (left panel, averages of 10 presentations), 250 Hz (middle panel, averages of 9 presentations), and 150 Hz (right panel, averages of 8 presentations). Filtering at still higher frequencies did not materially change twitch durations or amplitudes from those observed for filtering at 500 Hz. Fiber-base excursions were varied from 57 to 400 nm in 57 nm increments; the fiber’s stiffness was 293 μN · m−1. B, Twitch amplitude is plotted as a function of hair bundle displacement for the three families of variable-amplitude responses shown in A. Twitch amplitude decreased with greater rise time; in addition, the bundle displacement that elicited the largest twitch increased with the rise time. Because repetition of the stimuli elicited similar responses, it is improbable that fiber drift accounted for the observed results. The half-power frequencies of stimulus filtering were 500 Hz (□), 250 Hz (x), and 150 Hz (+).
Fig. 7.
The effect of holding position on twitches.A, By application of static offsets to the attached fiber, a hair bundle’s holding position was adjusted by the amounts shown below the traces. Identical, 140 nm displacement pulses were then applied to the fiber’s base. Although twitches occurred for a range of holding positions around the bundle’s resting position, they were suppressed by holding the bundle far in the positive or negative direction. Each trace is the average response to 8–10 stimulus presentations; the fiber’s stiffness was 272 μN · m−1. B, The mean twitch amplitudes of responses resulting from 140 nm stimulus pulses are plotted as a function of the bundle’s holding position. No twitches occurred from holding positions still more positive or negative than those shown. C, Adjustment of a hair bundle’s holding position caused twitches to become several cycles of damped mechanical oscillation. The bundle offsets for the three response families are shown to the bottom left of the respective records. Traces are the averages of 10–13 wavetrains of fiber-base excursions that were uniformly spaced between −300 and 300 nm. The fiber’s stiffness was 623 μN · m−1. The distance calibration bar at the left applies to_A_, the bar at the _right_applies to C, and the temporal calibration bar (middle) applies to both.
Fig. 8.
Fatigue and potentiation of the twitch response.A, Seven pairs of equal-amplitude and equal-duration displacement pulses were delivered to a stimulus fiber’s base with various delays between the pulses’ commencements. The twitch ensuing from the second stimulus was partially suppressed for the smallest intervals, then became slightly exaggerated with larger intervals. The delay between successive pairs of pulses was 150 msec; the results of 10 wave trains were averaged. The fiber of stiffness 535 μN · m−1 was displaced by 200 nm at its base. The displacement monitor’s output was filtered at 7 kHz and sampled by the computer at 14 kHz. B, The amplitude of the twitch elicited by the second pulse grew with the delay between the pulses’ onsets. The mean of the control response is shown with its SD.
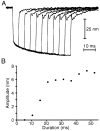
Fig. 9.
Development of the twitch during negative stimulation. A, A hair bundle was subjected to 10 stimuli of equal size, a fiber-base displacement of −300 nm, but differing durations. The amplitude of the twitch at the displacement’s conclusion grew monotonically with the duration of the stimulus. The delay between successive stimuli was 300 msec; the results of 14 stimulus wavetrains were averaged. The displacement monitor’s output was filtered at 3.5 kHz and communicated to the computer at 10 kHz. The fiber’s stiffness was 310 μN · m−1.B, A plot of twitch amplitudes from _A_against the durations of negative pulses demonstrates the gradual development of the capacity for twitching. Note that the time course of twitch capacitation approximately corresponds to that of mechanical adaptation.
Fig. 11.
Relation of twitches to the sensitivity of mechanoelectrical transduction. A, Variable-amplitude stimuli, applied to the base of a stimulus fiber, the tip of which was attached to a hair bundle, elicited a family of receptor potentials. The corresponding mechanical traces for this cell occur in Figure5_A_. B, While the amplitude of the receptor potential (□) grew monotonically with the stimulus size, the twitch’s amplitude (•) peaked for a bundle displacement near 40 nm.C, The twitch’s amplitude (•) is plotted against bundle displacement for another hair cell. As a measure of the sensitivity of mechanoelectrical transduction, the plot includes the derivative of the receptor potential’s slope as a function of bundle displacement (□). The initial slope of the receptor potential was determined by measuring the increment in electrical response between successive points separated by a 400 μsec sampling interval.D, The relation between the estimated sensitivity of mechanoelectrical transduction and twitch amplitude is approximately linear; the minimal squared error line through the origin is associated with a correlation coefficient r = 0.74. A represents the results from 15 step displacements uniformly spaced from −400 to 400 nm; the results from 10 stimulus wave trains were averaged. The fiber’s stiffness was 293 μN · m−1, and the cell’s resting potential was −63 mV.
Fig. 12.
Spontaneous twitching by hair bundles.A, Rapid, positively directed twitches of amplitudes as great as 30 nm were clearly distinct from the hair bundle’s RMS Brownian motion of ∼2 nm. The propensity to twitch depended on a bundle’s holding position, which is shown to the lower left of each trace. B, The same bundle’s rate of twitching was also sensitive to brief bundle displacements. Especially during negative displacement pulses, twitching required tens of milliseconds to resume. The bundle offsets were produced by 13 fiber base excursions that were uniformly varied in amplitude from −350 to 350 nm. The fiber’s stiffness was 310 μN · m−1.C, In another hair cell, spontaneous twitching of the hair bundle (top trace) was roughly synchronous with transient membrane depolarizations (bottom trace); the electrical responses may have been slightly delayed by the filtering effect of the membrane’s time constant. The responses were elicited by a 60 nm base displacement of a fiber of stiffness 753 μN · m−1; the cell’s resting potential was −45 mV. The time calibrations of B and C are identical.
Similar articles
- Active Biomechanics of Sensory Hair Bundles.
Bozovic D. Bozovic D. Cold Spring Harb Perspect Med. 2019 Nov 1;9(11):a035014. doi: 10.1101/cshperspect.a035014. Cold Spring Harb Perspect Med. 2019. PMID: 30323015 Free PMC article. Review. - Compressive nonlinearity in the hair bundle's active response to mechanical stimulation.
Martin P, Hudspeth AJ. Martin P, et al. Proc Natl Acad Sci U S A. 2001 Dec 4;98(25):14386-91. doi: 10.1073/pnas.251530498. Epub 2001 Nov 27. Proc Natl Acad Sci U S A. 2001. PMID: 11724944 Free PMC article. - Adaptive shift in the domain of negative stiffness during spontaneous oscillation by hair bundles from the internal ear.
Le Goff L, Bozovic D, Hudspeth AJ. Le Goff L, et al. Proc Natl Acad Sci U S A. 2005 Nov 22;102(47):16996-7001. doi: 10.1073/pnas.0508731102. Epub 2005 Nov 15. Proc Natl Acad Sci U S A. 2005. PMID: 16287969 Free PMC article. - Unifying the various incarnations of active hair-bundle motility by the vertebrate hair cell.
Tinevez JY, Jülicher F, Martin P. Tinevez JY, et al. Biophys J. 2007 Dec 1;93(11):4053-67. doi: 10.1529/biophysj.107.108498. Epub 2007 Aug 17. Biophys J. 2007. PMID: 17704173 Free PMC article. - Mechanoelectrical transduction by hair cells of the bullfrog's sacculus.
Hudspeth AJ. Hudspeth AJ. Prog Brain Res. 1989;80:129-35; discussion 127-8. doi: 10.1016/s0079-6123(08)62206-2. Prog Brain Res. 1989. PMID: 2699361 Review.
Cited by
- Review of chaos in hair-cell dynamics.
Faber J, Bozovic D. Faber J, et al. Front Neurol. 2024 Jul 10;15:1444617. doi: 10.3389/fneur.2024.1444617. eCollection 2024. Front Neurol. 2024. PMID: 39050124 Free PMC article. Review. - Dynamics of freely oscillating and coupled hair cell bundles under mechanical deflection.
Fredrickson-Hemsing L, Strimbu CE, Roongthumskul Y, Bozovic D. Fredrickson-Hemsing L, et al. Biophys J. 2012 Apr 18;102(8):1785-92. doi: 10.1016/j.bpj.2012.03.017. Biophys J. 2012. PMID: 22768934 Free PMC article. - Active Biomechanics of Sensory Hair Bundles.
Bozovic D. Bozovic D. Cold Spring Harb Perspect Med. 2019 Nov 1;9(11):a035014. doi: 10.1101/cshperspect.a035014. Cold Spring Harb Perspect Med. 2019. PMID: 30323015 Free PMC article. Review. - The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells.
Beurg M, Nam JH, Crawford A, Fettiplace R. Beurg M, et al. Biophys J. 2008 Apr 1;94(7):2639-53. doi: 10.1529/biophysj.107.123257. Epub 2008 Jan 4. Biophys J. 2008. PMID: 18178649 Free PMC article. - Thermal Excitation of the Mechanotransduction Apparatus of Hair Cells.
Azimzadeh JB, Fabella BA, Kastan NR, Hudspeth AJ. Azimzadeh JB, et al. Neuron. 2018 Feb 7;97(3):586-595.e4. doi: 10.1016/j.neuron.2018.01.013. Neuron. 2018. PMID: 29395911 Free PMC article.
References
- Art JJ, Crawford AC, Fettiplace R (1986) A method for measuring cellular movements less than the wavelength of light. J Physiol (Lond) 371:18P.
- Benser ME (1995) Characterization of active hair-bundle motion and its effect on the mechanics of organs of the acousticolateralis system. PhD thesis, University of Texas at Arlington.
- Benser ME, Issa NP, Hudspeth AJ. Hair-bundle stiffness dominates the elastic reactance to otolithic-membrane shear. Hear Res. 1993;68:243–252. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources