Mobility of synaptic vesicles in nerve endings monitored by recovery from photobleaching of synaptic vesicle-associated fluorescence - PubMed (original) (raw)
Mobility of synaptic vesicles in nerve endings monitored by recovery from photobleaching of synaptic vesicle-associated fluorescence
K Kraszewski et al. J Neurosci. 1996.
Abstract
In nerve terminals, synaptic vesicles form large clusters anchored to the presynaptic plasmalemma. Recently, FM1-43 photobleaching experiments carried out a frog motor end-plates demonstrated lack of lateral intermixing of synaptic vesicles within clusters, even during sustained nerve terminal stimulation (Henkel and Betz, 1995; Henkel et al., 1996b). We now have investigated the mobility of synaptic vesicle membranes during the endocytic limb of their exo-endocytic cycle. To this aim, we have carried out photobleaching experiments on nerve terminals of hippocampal neurons prelabeled with CY3-conjugated antibodies directed against lumenal epitopes of synaptotagmin I. This conjugate is taken up specifically by synaptic vesicle membranes during endocytosis and then is recovered in newly formed synaptic vesicles. Using this method, we show that synaptic vesicle membranes intermix after endocytosis. Staurosporine, which at hippocampal synapses partially inhibits unloading of FM1-43, but does not block uptake of antibody probes, prevents this intermixing. Our results indicate that synaptic vesicle docking and/or fusion with the plasmalemma correlate with the release of their membranes from a restraining matrix that hinders their lateral mobility. They suggest that membrane intermediates involved in synaptic vesicle reformation interact with a distinct, highly dynamic cytoskeleton and that newly formed synaptic vesicles are recaptured at random within vesicle clusters. Staurosporine, by inhibiting mobility within the terminal, may favor recapture of new vesicles near sites of endocytosis.
Figures
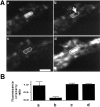
Fig. 1.
Effect of depolarization on the recovery from photobleaching in nerve terminals prelabeled with CY3-Sytlum-Abs. A, Sequential confocal microscopy images of the segment of a dendrite surrounded by axo-dendritic boutons. a, Presynaptic bouton after incubation with CY3-Sytlum-Abs for 10 min in KRH/high K+. The fluorescent area surrounded by a dotted white line represents a presynaptic terminal filled with fluorescent synaptic vesicles. The same field is shown in_b_ after complete laser-induced spot photobleaching of the portion of the terminal indicated by an arrow. The same field is shown in c after a second 3 min depolarization with KRH/high K+, and in d_after an incubation for 5 min with FM1-43 in KRH/high K+. The last incubation represents a control to show that the bleached nerve terminal still is fully viable and takes up FM1-43. A partial global loss of CY3 fluorescence from one field to the next is attributable to fluorescence decay during image collection. Note that in c, the residual fluorescence spreads to the whole nerve terminal, resulting in a partial recovery of fluorescence in the photobleached area. Scale bar, 2 μm. B, Quantification of results obtained from the experiment shown in_A and from two additional similar experiments.Bars represent ratios of fluorescence intensity between the control region of the terminal and the region subjected to photobleaching. Fluorescence intensity was measured with a confocal microscope on 0.16 μm2 (36 pixels) fields corresponding to the central areas of each of the two nerve terminal regions. Error bars represent SD.
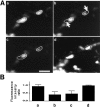
Fig. 2.
Photobleached areas in FM1-43-labeled nerve terminals do not recover from photobleaching after depolarization.A, Sequential confocal microscopy images of nerve terminals labeled with FM1-43. The same field is shown in_a_ after loading with FM1-43 in KRH/high K+for 10 min, in b after photobleaching of one nerve terminal, in c after 3 min depolarization with KRH/high K+, and in d after a new loading with FM1-43 in KRH/high K+. Note that in c, the unbleached part is significantly dimmer than in b(attributable to FM1-43 release with exocytosis and to previous image collection), but there is no diffusion of fluorescent signal from the unbleached to the bleached portion of the terminal. Scale bar, 2 μm.B, Quantification of results obtained from the experiment shown in A and from two additional similar experiments (see legend to Fig. 1_B_).
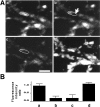
Fig. 3.
Staurosporine blocks depolarization-induced recovery from photobleaching in neurons labeled with CY3-Sytlum-Abs. A, Sequential confocal microscopy images of nerve terminals labeled with CY3-Sytlum-Abs. Nerve terminals were incubated for 1 hr with staurosporine (2 μ
m
) and then exposed to CY3-Sytlum-Abs for 10 min in KRH/high K+. The same field is shown in a at the end of CY3-Sytlum-Ab loading, in b after photobleaching of two nerve terminals (arrow), in_c_ after 3 min depolarization with KRH/high K+, and in d after loading with FM1-43 for 5 min in KRH/high K+ to show that the bleached nerve terminals still are fully viable and take up the dye. Note that in_c_, the residual fluorescence does not spread to the bleached area of nerve terminals with depolarization. Scale bar, 2 μm. B, Quantification of results obtained from the experiment shown in A and from two additional similar experiments (see legend to Fig.1_B_).
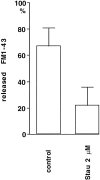
Fig. 4.
Staurosporine blocks the unloading of FM1-43. Neurons were incubated 1 hr in KRH with or without staurosporine (2 μ
m
), loaded with FM1-43 in KRH/high K+ for 10 min, rinsed in KRH, and then reexposed to KRH/high K+ in the absence of FM1-43 to unload the dye. Bars indicate ratios between the fluorescence intensity observed on individual boutons at the end of the load and after the unloading period. Fluorescence intensity was measured with a chilled CCD camera. Error bars represent SEM.
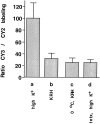
Fig. 5.
Quantitative analysis of exo-endocytosis based on the comparative uptake of CY3-Sytlum-Abs and CY2-Sytlum-Abs. Neurons first were incubated with CY2-Sytlum-Abs for 10 min in KRH/high K+. Then after one additional hour in KRH, which contained tetanus toxin (50 n
m
) in the case of test condition d, they were incubated with CY3-Sytlum-Abs for 10 min under the following test conditions: KRH/high K+ in a, KRH in b, KRH at 0°C in c, and KRH/high K+ in d. Bars represent the ratio between the CY3 and CY2 fluorescence (which was measured at the end of the experiments with a chilled CCD camera) expressed as percentages of the CY3/CY2 ratio observed in the test condition a. Error bars represent SEM.
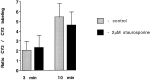
Fig. 6.
Staurosporine does not block the uptake of CY3-Sytlum-Abs. Neurons were incubated in KRH/high K+ containing CY2-Sytlum-Abs for 10 min, then for 1 hr in KRH with or without staurosporine (2 μ
m
), and finally for 3 or 10 min with KRH/high K+ containing CY3-Sytlum-Abs. Bars represent ratios between the CY2 and CY3 fluorescence at the end of the experiment.
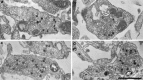
Fig. 7.
Staurosporine does not block uptake of both HRP/Sytlum-Abs and HRP in nerve terminals of hippocampal neurons. Electron micrographs of nerve terminals from cultures incubated first for 1 hr in KRH with (b,d) or without (a, c) staurosporine (2 μ
m
), then for 10 min with HRP/Sytlum-Abs (a, b) or HRP (c, d) in KRH/high K+. Note presence of HRP-labeled synaptic vesicles in all conditions.
Similar articles
- Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin.
Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. Kraszewski K, et al. J Neurosci. 1995 Jun;15(6):4328-42. doi: 10.1523/JNEUROSCI.15-06-04328.1995. J Neurosci. 1995. PMID: 7540672 Free PMC article. - Interaction of stoned and synaptotagmin in synaptic vesicle endocytosis.
Fergestad T, Broadie K. Fergestad T, et al. J Neurosci. 2001 Feb 15;21(4):1218-27. doi: 10.1523/JNEUROSCI.21-04-01218.2001. J Neurosci. 2001. PMID: 11160392 Free PMC article. - Effects of staurosporine on exocytosis and endocytosis at frog motor nerve terminals.
Becherer U, Guatimosim C, Betz W. Becherer U, et al. J Neurosci. 2001 Feb 1;21(3):782-7. doi: 10.1523/JNEUROSCI.21-03-00782.2001. J Neurosci. 2001. PMID: 11157064 Free PMC article. - Use of fluorescent probes to follow membrane traffic in nerve terminals.
Guatimosim C, Romano-Silva MA, Gomez MV, Prado MA. Guatimosim C, et al. Braz J Med Biol Res. 1998 Nov;31(11):1491-500. doi: 10.1590/s0100-879x1998001100018. Braz J Med Biol Res. 1998. PMID: 9921287 Review. - Mechanisms of synaptic vesicle recycling illuminated by fluorescent dyes.
Cousin MA, Robinson PJ. Cousin MA, et al. J Neurochem. 1999 Dec;73(6):2227-39. doi: 10.1046/j.1471-4159.1999.0732227.x. J Neurochem. 1999. PMID: 10582580 Review.
Cited by
- Subdiffractional tracking of internalized molecules reveals heterogeneous motion states of synaptic vesicles.
Joensuu M, Padmanabhan P, Durisic N, Bademosi AT, Cooper-Williams E, Morrow IC, Harper CB, Jung W, Parton RG, Goodhill GJ, Papadopulos A, Meunier FA. Joensuu M, et al. J Cell Biol. 2016 Oct 24;215(2):277-292. doi: 10.1083/jcb.201604001. Epub 2016 Oct 24. J Cell Biol. 2016. PMID: 27810917 Free PMC article. - Antibody-driven capture of synaptic vesicle proteins on the plasma membrane enables the analysis of their interactions with other synaptic proteins.
Richter KN, Patzelt C, Phan NTN, Rizzoli SO. Richter KN, et al. Sci Rep. 2019 Jun 25;9(1):9231. doi: 10.1038/s41598-019-45729-4. Sci Rep. 2019. PMID: 31239503 Free PMC article. - Importance of Full-Collapse Vesicle Exocytosis for Synaptic Fatigue-Resistance at Rat Fast and Slow Muscle Neuromuscular Junctions.
Rudling JE, Drever BD, Reid B, Bewick GS. Rudling JE, et al. Int J Mol Sci. 2018 Jul 2;19(7):1936. doi: 10.3390/ijms19071936. Int J Mol Sci. 2018. PMID: 30004407 Free PMC article. - Taking a back seat: synaptic vesicle clustering in presynaptic terminals.
Pechstein A, Shupliakov O. Pechstein A, et al. Front Synaptic Neurosci. 2010 Sep 15;2:143. doi: 10.3389/fnsyn.2010.00143. eCollection 2010. Front Synaptic Neurosci. 2010. PMID: 21423529 Free PMC article. - Properties of fast endocytosis at hippocampal synapses.
Kavalali ET, Klingauf J, Tsien RW. Kavalali ET, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Feb 28;354(1381):337-46. doi: 10.1098/rstb.1999.0385. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212482 Free PMC article.
References
- Bennett MK, Scheller RH. A molecular description of synaptic vesicles membrane trafficking. Annu Rev Biochem. 1994;63:63–100. - PubMed
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources