Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus - PubMed (original) (raw)
Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus
A H Gazzaley et al. J Neurosci. 1996.
Abstract
Estradiol treatment increases the number of NMDA receptor binding sites, and changes evoked synaptic currents in a manner consistent with a steroid-induced functional enhancement of NMDA receptors in rat hippocampus. In this study, we investigate the cellular mechanisms of estradiol-induced NMDA receptor regulation at the protein and mRNA levels in ovariectomized rats treated with ovarian steroids using immunocytochemical and in situ hybridization techniques. Confocal laser scanning microscopy was used to quantify alterations in immunofluorescence intensity levels of NMDAR1 subunit proteins within neuronal somata and dendrites of discrete hippocampal fields, whereas in parallel, in situ hybridization was used to examine NMDAR1 mRNA levels in corresponding hippocampal regions. The data indicate that estradiol treatment in ovariectomized rats significantly increases immunofluorescence intensity levels in comparison with nonsteroid treated ovariectomized rats within the somata and dendrites of CA1 pyramidal cells and, to a lesser extent, within the granule cell somata of the dentate gyrus. In contrast, such alterations in immunofluorescence intensity occur without concomitant changes in mRNA hybridization levels. Thus, these data suggest that estradiol modulates NMDA receptor function via post-transcriptional regulation of the NMDAR1 subunit protein. The increase in immunofluorescence intensity may reflect an increase in the concentration of the subunit protein, which could account for estrogen-induced changes in pharmacological and physiological properties of the NMDA receptor.
Figures
Fig. 1.
CLSM images of NMDAR1 immunolabeled CA1 pyramidal cell somata (A, B) and dendrites (B, C) before (A,C) and after (B, D) intensity thresholding, as indicated by the blue color overlay. After the thresholding procedure, all unlabeled portions of the field (in_blue_) have no contribution to the average intensity of the field.
Fig. 2.
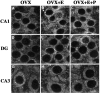
Examples of CLSM images of NMDAR1 immunolabeled somata in CA1 (A_–_C), the dentate gyrus (D_–_F), and CA3 (G_–_I) of OVX (A,D, G), OVX+E (B,E, H), and OVX+E+P (C, F, I) rats. Note the presence of punctate staining within the cytoplasm surrounding the unlabeled nuclei (see Discussion). When comparing the CA1 fields, an increase in the somatic intensity of staining is evident in the OVX+E and OVX+E+P rats (B, C) as compared with the OVX rats (A). This increase in the steroid-treated ovariectomized rats is also apparent in the dentate gyrus (E, F compared with_D_), although to a lesser extent. There is no obvious difference in intensity levels among the three groups in the CA3 field (G_–_I). DG, Dentate gyrus; OVX, ovariectomized rats; OVX+E, estradiol-treated ovariectomized rats; OVX+E+P, estradiol plus progesterone-treated ovariectomized rats. Scale bars, 10 μm.
Fig. 3.
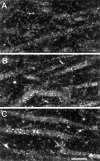
Examples of CLSM images of NMDAR1 immunolabeled dendrites in the CA1 subfield of an OVX rat (A), an OVX+E rat (B), and an OVX+E+P rat (C). Note the presence of punctate staining within the cytoplasm of the dendritic segments (see Discussion) and the increased intensity of staining within the CA1 dendrites of the steroid-treated ovariectomized rats (B,C) rats compared with the nonsteroid-treated ovariectomized rats (A). Scale bar, 10 μm.
Fig. 4.
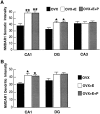
Bar graphs depicting NMDAR1 immunofluorescence intensity measurements in the somata (A) and dendrites (B) of the CA1, DG, and CA3 fields of the hippocampus. For somatic intensity measurements (A), there is a significant increase in both CA1 and the dentate gyrus when comparing OVX+E and OVX+E+P rats with OVX rats. Based on the results of the somatic intensity measurements (A), the CA1 and the DG dendritic fields were quantified (B). In_B_, note that there are significant increases only in the dendritic intensity measurements of steroid-treated rats as compared with the OVX rats in the CA1 subfield, although there is a trend toward increase in the dentate gyrus. Values represent mean ± SEM for six OVX rats and five OVX+E and OVX+E+P rats (24 measurements per rat). *p < 0.05, **p < 0.0001 compared with OVX group; ANOVA and Sheffe’s test.
Fig. 5.
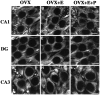
Examples of CLSM images of MAP2-immunolabeled somata in CA1 (A_–_C), the dentate gyrus (D_–_F), and CA3 (G_–_I) of OVX rats (A, D, G), OVX+E rats (B, E, H), and OVX+E+P rats (C, F,I). Note that there are no obvious differences in intensity levels when comparing the different experimental groups within any hippocampal subfield. Scale bars, 10 μm.
Fig. 6.
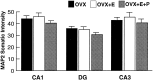
Bar graphs depicting MAP2 immunofluorescence intensity measurements in the somata of the CA1, dentate gyrus, and CA3 fields of the hippocampus. Quantitative analysis revealed no statistically significant differences among the three groups when comparing within a hippocampal field. Values represent mean ± SEM for six OVX rats, five OVX+E rats, and OVX+E+P rats (24 measurements per rat).
Fig. 7.
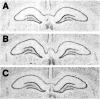
Photomicrographs of film autoradiograms show NMDAR1 mRNA hybridization in the hippocampus of an OVX rat (A), an OVX+E rat (B), and an OVX+E+P rat (C). Note that there is no overt difference in hybridization distribution or intensity in the hippocampal subfields among rats from different treatment groups.
Fig. 8.
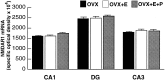
Bar graphs depicting the quantification of NMDAR1 mRNA labeling in the CA1, dentate gyrus, and CA3 subfields of the hippocampus. There were no statistically significant differences in optical density measurements in any of the subfields among the three groups. Values represent mean ± SEM for nine OVX rats, nine OVX+E, and nine OVX+E+P rats.
Similar articles
- Differential subcellular regulation of NMDAR1 protein and mRNA in dendrites of dentate gyrus granule cells after perforant path transection.
Gazzaley AH, Benson DL, Huntley GW, Morrison JH. Gazzaley AH, et al. J Neurosci. 1997 Mar 15;17(6):2006-17. doi: 10.1523/JNEUROSCI.17-06-02006.1997. J Neurosci. 1997. PMID: 9045729 Free PMC article. - Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance.
El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. El-Bakri NK, et al. J Cell Mol Med. 2004 Oct-Dec;8(4):537-44. doi: 10.1111/j.1582-4934.2004.tb00478.x. J Cell Mol Med. 2004. PMID: 15601582 Free PMC article. - Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain.
Cyr M, Ghribi O, Di Paolo T. Cyr M, et al. J Neuroendocrinol. 2000 May;12(5):445-52. doi: 10.1046/j.1365-2826.2000.00471.x. J Neuroendocrinol. 2000. PMID: 10792584 - Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors.
Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Cyr M, et al. Brain Res Brain Res Rev. 2001 Nov;37(1-3):153-61. doi: 10.1016/s0165-0173(01)00115-1. Brain Res Brain Res Rev. 2001. PMID: 11744083 Review.
Cited by
- Maturation profile of inferior olivary neurons expressing ionotropic glutamate receptors in rats: role in coding linear accelerations.
Li C, Han L, Ma CW, Lai SK, Lai CH, Shum DK, Chan YS. Li C, et al. Brain Struct Funct. 2013 Jul;218(4):833-50. doi: 10.1007/s00429-012-0432-3. Epub 2012 Jun 16. Brain Struct Funct. 2013. PMID: 22706760 Free PMC article. - Susceptibility to Calcium Dysregulation during Brain Aging.
Kumar A, Bodhinathan K, Foster TC. Kumar A, et al. Front Aging Neurosci. 2009 Nov 27;1:2. doi: 10.3389/neuro.24.002.2009. eCollection 2009. Front Aging Neurosci. 2009. PMID: 20552053 Free PMC article. - Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling.
McEwen BS. McEwen BS. Ann N Y Acad Sci. 2010 Sep;1204 Suppl(Suppl):E38-59. doi: 10.1111/j.1749-6632.2010.05568.x. Ann N Y Acad Sci. 2010. PMID: 20840167 Free PMC article. Review. - Neuroprotection against excitotoxic brain injury in mice after ovarian steroid depletion.
Schauwecker PE, Wood RI, Lorenzana A. Schauwecker PE, et al. Brain Res. 2009 Apr 10;1265:37-46. doi: 10.1016/j.brainres.2009.02.023. Epub 2009 Feb 21. Brain Res. 2009. PMID: 19236850 Free PMC article. - The influences of reproductive status and acute stress on the levels of phosphorylated mu opioid receptor immunoreactivity in rat hippocampus.
Gonzales KL, Chapleau JD, Pierce JP, Kelter DT, Williams TJ, Torres-Reveron A, McEwen BS, Waters EM, Milner TA. Gonzales KL, et al. Front Endocrinol (Lausanne). 2011 Aug 19;2(18):18. doi: 10.3389/fendo.2011.00018. Front Endocrinol (Lausanne). 2011. PMID: 22468144 Free PMC article.
References
- Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. - PubMed
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
- Buterbaugh GC, Hudson GM. Estradiol replacement to female rats facilitates dorsal hippocampal but not ventral hippocampal kindled seizure acquisition. Exp Neurol. 1991;111:55–64. - PubMed
- Dodge DE, Rucker RB, Singh G, Plopper CG. Quantitative comparison of intracellular concentration and volume of Clara cell 10 KD protein in rat bronchi and bronchioles based in laser scanning confocal microscopy. J Histochem Cytochem. 1993;41:1171–1183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS007080/NS/NINDS NIH HHS/United States
- R37 AG006647/AG/NIA NIH HHS/United States
- NS-07080/NS/NINDS NIH HHS/United States
- R01 AG006647/AG/NIA NIH HHS/United States
- NS-30105/NS/NINDS NIH HHS/United States
- AG-06647/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous