Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract - PubMed (original) (raw)
Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract
E F Grady et al. J Neurosci. 1996.
Abstract
Understanding the physiological role of tachykinins requires precise cellular and subcellular localization of their receptors. We raised antisera by immunizing rabbits with peptides corresponding to portions of the intracellular tails of the rat neurokinin 1, 2, and 3 receptors (NK1-R, NK2-R, NK3-R). Receptors were localized by immunofluorescence and confocal microscopy. NK1-R, NK2-R, and NK3-R were detected at the plasma membrane of transfected cells with minimal intracellular stores. Staining was abolished by preabsorption of the antisera with the peptides used for immunization. Nontransfected cells were unstained. Each antiserum only stained cells transfected with the appropriate receptor and did not stain cells transfected with the other receptors. Therefore, the antisera are specific and do not cross-react with other neurokinin receptors. We examined the distribution of the neurokinin receptors in the gastrointestinal tract of the rat. NK1-R was detected in myenteric and submucosal neurons and in interstitial cells of Cajal. NK2-R was localized to circular and longitudinal muscle cells and to nerve endings in the plexuses. NK3-R was detected in numerous myenteric and submucosal neurons. Some neurons expressed both NK1-R and NK3-R. Receptors were detected at the plasma membrane and in endosomes. Cells expressing the receptors were closely associated with tachykinin-containing nerve fibers. Thus, NK1-R and NK3-R mediate neurotransmission by tachykinins within enteric nerve plexuses, and NK1-R and NK2-R mediate the effects of tachykinins on interstitial and smooth muscle cells, respectively.
Figures
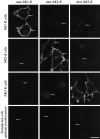
Fig. 1.
Confocal photomicrographs showing localization of NK1-R, NK2-R, and NK3-R in cell lines. The top horizontal panels show KNRK-NK1-R cells, the second horizontal panels show CHO-NK2-R cells, and the third horizontal panels show KNRK-NK3-R cells. The bottom horizontal panels show transfected cell lines expressing NK1-R (left), NK2-R (middle), and NK3-R (right). Cells were incubated with NK1-R antiserum (#94168, left vertical panels), NK2-R antiserum (#94179,middle vertical panels), and NK3-R antiserum (#94192,right vertical panels), followed by Texas Red or FITC-conjugated secondary antibodies. The bottom horizontal panels show transfected cells that were incubated with antisera preabsorbed with the peptides used for immunization. The_arrows_ indicate that the antisera stain the plasma membrane of cells expressing the appropriate receptor and do not cross-react with cells expressing the other receptors. The_arrows_ in the _bottom horizontal panels_indicate that staining was abolished by preabsorption with the receptor fragment used for immunization. Scale bar, 20 μm.
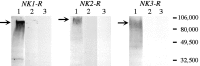
Fig. 2.
Western blot analysis using_NK1-R_ antiserum (#94168), NK2-R antiserum (#94179), and NK3-R antiserum (#94192). Each lane contains 10 μg of protein. Lane 1, Transfected cells expressing NK1-R, NK2-R, or NK3-R. Lane 2, Transfected cells expressing NK1-R, NK2-R, or NK3-R, with antisera preabsorbed with receptor fragment. Lane 3, Nontransfected cells. The_arrows_ indicate the major bands that were detected in transfected cells.
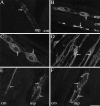
Fig. 3.
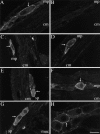
Confocal photomicrographs showing localization of NK1-R (A_–_E) and NK3-R (F) in tissue sections and whole mounts. Tissues were incubated with NK1-R antiserum #94168 or NK3-R antiserum AP951.A, Section of antrum, showing localization of NK1-R in myenteric neurons. B, Section of jejunum, showing localization of NK1-R in myenteric neurons and interstitial cells of Cajal. C, Whole mount of the myenteric plexus of the ileum. D, Whole mount of interstitial cells of Cajal of the ileum. The arrows indicate prominent staining of the plasma membrane and endosomes. E, The sum of five optical sections of jejunum, collected at 0.36 μm intervals, showing localization of NK1-R in myenteric neurons and in interstitial cells of Cajal. F, An adjacent section to E (8 μm apart), the sum of five optical sections showing localization of NK3-R. The same neurons expressing the NK1-R express the NK3-R, whereas the interstitial cells are unstained.B_–_E show NK1-R-immunoreactive endosomes.mp, Myenteric plexus; cm, circular muscle. Scale bar (shown in F):A_–_D, 20 μm; E,F, 13 μm.
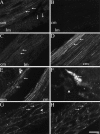
Fig. 4.
Confocal photomicrographs showing localization of NK2-R in tissue sections and whole mounts. Tissues were incubated with NK2-R antiserum #94179. A, Section of circular and longitudinal muscle layers of the duodenum. B, Serial section from A in which the antiserum is preabsorbed with 1 μm of the receptor fragment that was used for immunization.C, Section of circular and longitudinal muscle layers of the fundus. D, Section of circular muscle layer of the colon. E, Section of muscularis mucosa and circular muscle layer of the colon. The muscularis mucosa is identified by_arrows_. F, The sum of 10 optical sections, collected at 0.54 μm intervals, of a whole mount of the submucosal plexus of the ileum showing a stained nerve ending.G, Section of circular muscle of the fundus. The_arrows_ indicate prominent staining of the plasma membrane. The arrowhead indicates endosomes.H, Image of the same optical section shown in_G_, showing staining with Cell Tracker CM-DiI to outline the cell surface. mp, Myenteric plexus;cm, circular muscle; lm, longitudinal muscle; mm, muscularis mucosa. Scale bar (shown in_H_): A_–_E, 20 μm;F, 10 μm; G, H, 5 μm.
Fig. 5.
Confocal photomicrographs showing localization of NK3-R in tissue sections and whole mounts. Tissues were incubated with NK3-R antiserum #94192 in A,B, D, and E, or AP951 in_C_, F, G, and_H_. A, Section of duodenum, showing localization of NK3-R in myenteric neurons. B, Serial section from A in which the antiserum is preabsorbed with 1 μ
m
of the receptor fragment that was used for immunization. C, Section of fundus, showing localization of NK3-R in myenteric neurons. D, Section of jejunum, showing localization of NK3-R in myenteric neurons. E, Section of ileum, showing localization of NK3-R in submucosal neurons.F, Section of colon, showing localization of NK3-R in myenteric neurons. G, Section of colon, showing localization of NK3-R in submucosal neurons. H, Whole mount of the myenteric plexus of the ileum showing NK3-R at the plasma membrane and in endosomes. The arrows indicate prominent staining of the plasma membrane and endosomes. mp, Myenteric plexus; sp, submucosal plexus;cm, circular muscle; lm, longitudinal muscle; muc, mucosa. Scale bar (shown in_H_), 20 μm.
Fig. 6.
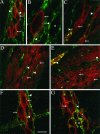
Confocal photomicrographs showing localization of neurokinin receptors (red, arrows) and tachykinins (green, arrowheads).A_–_C, NK1-R and tachykinins in whole mounts of the ileal myenteric plexus (A,B) and interstitial cells of Cajal (C). D, E, NK2-R and tachykinins in the gastric antrum. F,G, NK3-R and tachykinins in whole mounts of the ileal myenteric plexus. Cells expressing the neurokinin receptors (arrows) are closely associated with tachykinin containing nerve fibers (arrowheads). Not all neurons within a plexus express a given neurokinin receptor (asterisks), but all neurons are surrounded by tachykinin-containing fibers. Scale bar (shown in_F_): A, C,E_–_G, 20 μm; B, 10 μm;D, 30 μm.
Similar articles
- Postnatal development of NK1, NK2, and NK3 neurokinin receptors expression in the rat retina.
Oyamada H, Takatsuji K, Senba E, Mantyh PW, Tohyama M. Oyamada H, et al. Brain Res Dev Brain Res. 1999 Oct 20;117(1):59-70. doi: 10.1016/s0165-3806(99)00099-1. Brain Res Dev Brain Res. 1999. PMID: 10536233 - Effect of scyliorhinin I and synthetic scyliorhinin I derivatives at mammalian tachykinin NK1, NK2 and NK3 receptors.
Patacchini R, Quartara L, Rolka K, Zboinska J, Kupryszewski G, Maggi CA. Patacchini R, et al. Eur J Pharmacol. 1993 Dec 7;250(2):311-6. doi: 10.1016/0014-2999(93)90396-y. Eur J Pharmacol. 1993. PMID: 7509285 - Stable expression of high affinity NK1 (substance P) and NK2 (neurokinin A) receptors but low affinity NK3 (neurokinin B) receptors in transfected CHO cells.
Gether U, Marray T, Schwartz TW, Johansen TE. Gether U, et al. FEBS Lett. 1992 Jan 27;296(3):241-4. doi: 10.1016/0014-5793(92)80295-r. FEBS Lett. 1992. PMID: 1311270 - Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human.
Lecci A, Capriati A, Altamura M, Maggi CA. Lecci A, et al. Auton Neurosci. 2006 Jun 30;126-127:232-49. doi: 10.1016/j.autneu.2006.02.014. Epub 2006 Apr 17. Auton Neurosci. 2006. PMID: 16616700 Review. - Tachykinin receptors and receptor subtypes.
Patacchini R, Maggi CA. Patacchini R, et al. Arch Int Pharmacodyn Ther. 1995 Jan-Feb;329(1):161-84. Arch Int Pharmacodyn Ther. 1995. PMID: 7639617 Review.
Cited by
- cAMP signaling through protein kinase A and Epac2 induces substance P release in the rat spinal cord.
Chen W, McRoberts JA, Ennes HS, Marvizon JC. Chen W, et al. Neuropharmacology. 2021 May 15;189:108533. doi: 10.1016/j.neuropharm.2021.108533. Epub 2021 Mar 17. Neuropharmacology. 2021. PMID: 33744339 Free PMC article. - Endosomal endothelin-converting enzyme-1: a regulator of beta-arrestin-dependent ERK signaling.
Cottrell GS, Padilla BE, Amadesi S, Poole DP, Murphy JE, Hardt M, Roosterman D, Steinhoff M, Bunnett NW. Cottrell GS, et al. J Biol Chem. 2009 Aug 14;284(33):22411-22425. doi: 10.1074/jbc.M109.026674. Epub 2009 Jun 16. J Biol Chem. 2009. PMID: 19531493 Free PMC article. - Desensitization of the neurokinin-1 receptor (NK1-R) in neurons: effects of substance P on the distribution of NK1-R, Galphaq/11, G-protein receptor kinase-2/3, and beta-arrestin-1/2.
McConalogue K, Corvera CU, Gamp PD, Grady EF, Bunnett NW. McConalogue K, et al. Mol Biol Cell. 1998 Aug;9(8):2305-24. doi: 10.1091/mbc.9.8.2305. Mol Biol Cell. 1998. PMID: 9693383 Free PMC article. - Detection of naturally expressed receptors for gastrin-releasing peptide and tachykinins using cyanine 3-labelled neuropeptides.
Bunnett NW, Payan DG, Grady EF. Bunnett NW, et al. Histochem J. 1996 Nov;28(11):811-26. doi: 10.1007/BF02272154. Histochem J. 1996. PMID: 8968733 - Inflammation-induced abnormalities in the subcellular localization and trafficking of the neurokinin 1 receptor in the enteric nervous system.
Poole DP, Lieu T, Pelayo JC, Eriksson EM, Veldhuis NA, Bunnett NW. Poole DP, et al. Am J Physiol Gastrointest Liver Physiol. 2015 Aug 15;309(4):G248-59. doi: 10.1152/ajpgi.00118.2015. Epub 2015 Jul 2. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26138465 Free PMC article.
References
- Bergström L, Beaujouan JC, Torrens Y, Saffroy M, Glowinski J, Lavielle S, Chassaing G, Marquet A, D’Orleans-Juste P, Dion S, Regoli D. 3H-neurokinin A labels a specific tachykinin-binding site in the rat duodenal smooth muscle. Mol Pharmacol. 1987;32:764–771. - PubMed
- Bunnett NW, Dazin PF, Payan DG, Grady EF. Characterization of receptors using cyanine 3-labeled neuropeptides. Peptides. 1995;16:733–740. - PubMed
- Burcher E, Shults CW, Buck SH, Chase TN, O’Donohue TL. Autoradiographic distribution of substance K binding sites in rat gastrointestinal tract: a comparison with substance P. Eur J Pharmacol. 1984;102:561–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL024136/HL/NHLBI NIH HHS/United States
- R37 DK039957/DK/NIDDK NIH HHS/United States
- DK39957/DK/NIDDK NIH HHS/United States
- R56 DK043207/DK/NIDDK NIH HHS/United States
- DK43207/DK/NIDDK NIH HHS/United States
- R01 DK043207/DK/NIDDK NIH HHS/United States
- NS21710/NS/NINDS NIH HHS/United States
- R01 HD033024/HD/NICHD NIH HHS/United States
- R01 DK039957/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases