Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg - PubMed (original) (raw)
Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg
V Budnik et al. Neuron. 1996 Oct.
Abstract
Mutations of the tumor suppressor gene discs-large (dlg) lead to postsynaptic structural defects. Here, we report that mutations in dlg also result in larger synaptic currents at fly neuromuscular junctions. By selectively targeting DLG protein to either muscles or motorneurons using Gal-4 enhancer trap lines, we were able to rescue substantially the reduced postsynaptic structure in mutants. Rescue of the physiological defect was accomplished by presynaptic, but not postsynaptic targeting, consistent with our finding that miniature excitatory junctional currents were not changed in dlg mutants. These results suggest that DLG functions in the regulation of neurotransmitter release and postsynaptic structure. We propose that DLG is an integral part of a mechanism by which changes in both neurotransmitter release and synapse structure are accomplished during development and plasticity.
Figures
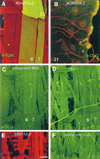
Figure 1. Targeted Expression of dlg in Postsynaptic Cells Results in DLG Localization to Type I Junctions
(A) Expression of Gal-4 in longitudinal muscles 6, 7, and 31 in the P[Gal-4] insertion line BG487 is detected by staining body wall muscles from progeny of the cross BG487 x UAS-LacZ with anti-βgal antibodies (green). All body wall muscles are double-labeled with rhodamine-conjugated phalloidin (red). (B) View of muscle 31 in a dlgm52;post-dlg larva, in which the Gal-4 strain BG487 has been used to target DLG expression in the muscle cell. This preparation has been double-labeled with anti-DLG (green) and anti-HRP (red), a nervous system-specific antibody. Note that DLG is targeted only to Type I boutons in muscle 31, even though this muscle is innervated by both Type I and Type II endings. (C) and (D) View of abdominal segment 2 in preparations stained with anti-DLG antibodies. DLG immunoreactivity at Type I boutons in muscles 6 and 7 in dlgm52/Df; post-dlg (C). Note that in contrast to wild type (D), strong immunoreactivity is only observed at muscles 6 and 7 at Type I boutons. (E) and (F) View of a late first instar body wall muscle segment. A BG57/UAS-LacZ preparation stained with anti-βgal antibodies (E), and a dlgm52/Df; UAS-dlg/+; BG57/+ preparation stained with anti-DLG antibodies (F). These and subsequent light micrographs are confocal images, projected from a Z-series. Scale bars, 40 µm (A); 8 µm (B); 70 µm (C and D); and 20 µm (E and F).
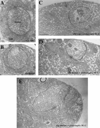
Figure 2. Regulation of SSR Size by dlg
(A) Electron micrograph of a Type Ib bouton in a wild-type larva, shows a normal SSR. (B) In a dlgm52/Df mutant larva, the SSR is underdeveloped. (C) Postsynaptically driven dlg (UAS-dlg/ BG487) causes the SSR to appear overdeveloped. (D) In a dlgm52/Df mutant larva, dlg has been driven in the postsynaptic cell using BG487 (dlgm52; post-dlg), and the mutant phenotype at the SSR has been partially rescued. (E) Similarly, in a dlgm52; pre-dlg, dlg has been driven in the presynaptic cells using sca-Gal-4, and the SSR appears normal. Scale bar, 0.8 µm.
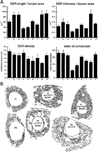
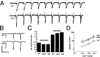
Figure 3. Morphometric Analysis of the SSR
(A) The normalized cross-sectional SSR length, normalized thickness, density, and index of convolution at Type Ib boutons are compared in nine genotypes as follows: in (1) wild type; (2) post-dlg (one copy UAS–dlg [UAS–dlg/BG487]); (3) post-dlg (two copies UAS–dlg [UAS–dlg/Y; UAS–dlg/BG487]); (4) dlgm52/Df; (5) dlgv59/Df; (6) dlg–post-dlg (dlgm52/ Df; UAS–dlg/BG487); (7) dlg–post-dlg (dlgm52/ Df; UAS–dlg/BG57); (8) dlg–pre-dlg(dlgv59/-Df; sca–Gal-4/UAS–dlg); and (9) dlg–pre-dlg (dlgm52/Df; UAS–dlg/BG380). (B) Representative SSR tracing of some of the genotypes used for the analysis in (A) is diagrammed. The number of boutons used for the morphometric analysis is as follows: wild type: 12 boutons, wild type; post-dlg (one copy of UAS–dlg)): 12 boutons, wild type; post-dlg (two copies of UAS–dlg): 11 boutons; _dlg_m52: 10 boutons; dlgv59: 10 boutons; dlg; post-dlg (BG487): 18 boutons; dlg; post-dlg (BG57): 10 boutons; dlg pre-dlg (sca–Gal-4): 12 boutons; dlg pre-dlg (BG380): 10 boutons.
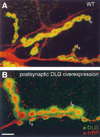
Figure 4. Postsynaptic dlg Overexpression Results in a Larger than Normal Immunoreactive Area around Type I Boutons
(A) and (B) Nerve endings in muscle 6 showing Type Ib and Type Is boutons in preparations double-stained with anti-DLG (green) and anti-HRP (red) antibodies. Note how Type Ib boutons (B) in a larva with three extra dlg gene doses (UAS-dlg/Y; BG487/UAS-dlg) have very extensive DLG immunoreactivity compared to wild type (A). Scale bar, 10 µm.
Figure 5. Evoked and Spontaneous Synaptic Currents Are Compared in Two dlg Mutant Alleles and in dlgm52/Df with Pre- and Postsynaptic DLG Targeting
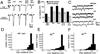
(A) Evoked currents were recorded in saline containing 1.5 mM Ca2+ in wild type, dlgv59/Df mutants (v59), dlgm52/Df mutants (m52), dlgm52/ Df; post-dlg, in which dlg expression is driven postsynaptically (post-DLG), and dlgm52/Df; UAS-dlg/sca-Gal-4, in which dlg expression is driven presynaptically (pre-DLG). Note that two classes of synaptic currents (low and suprathreshold EJCs) are observed in wild type and mutants and that their amplitude is increased in both dlg alleles. This abnormal phenotype is rescued in larvae with pre- but not postsynaptic expression. (B) A histogram plot of the average low and suprathreshold EJC amplitudes (mean ± SEM). (C) This panel shows examples of miniature EJCs in wild type, dlgv59/Df, and dlgm52/Df. (D)-(F) Distribution of miniature EJC amplitudes is compared between wild type (D), dlgv59/Df (E), and dlgm52/Df (F).
Figure 6. Short Term Plasticity of Wild-Type and dlg Mutant Synapses and Ca2+ Dependency of Quantal Content
(A) Train of EJCs in a wild type and dlgv59 mutant preparation stimulated at 10 Hz recorded in saline containing 1.5 mM Ca2+. Note that both wild-type and mutant EJCs depress under these conditions. (B) Train of EJCs in wild-type and dlgm52 preparations stimulated at 10 Hz recorded in saline containing 0.6 mM Ca2+. Note that both wild-type and mutant EJCs facilitate. (C) Histogram of the mean depression and facilitation index in wild-type and dlg mutant alleles. (D) Double log plot of Ca2+ concentration versus quantal content demonstrates that mutant EJCs remain significantly larger than wild type at different Ca2+ concentrations and that the Ca2+ dependency of EJCs is unchanged. These results were obtained from at least four muscle fibers at each Ca2+ concentration and in each genotype. Calibration bars at the left corner correspond to 80 nA and 120 ms in (A), and to 20 nA and 100 ms in (B).
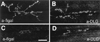
Figure 7. Targeting DLG to Presynaptic Endings
(A) and (B) Type I synaptic boutons in muscles 6 and 7 of a third instar BG380/UAS-LacZ preparation stained with anti-βgal antibodies (A), and of a third instar BG380/UAS– dlg sample stained with anti-DLG antibodies (B). Note that the boutons in (A) appear smaller than those in (B), probably owing to the differential localization of the antigens (cytoplasmic in the case of βgal, and membrane-associated in the case of DLG. (C) and (D) Type I synaptic boutons in muscles 6 and 7 of a late first instar sca-Gal-4/U_AS-LacZ_ stained with anti-βgal antibodies (C), and of a late first instar sca-Gal-4/U_AS-dlg_ sample stained with anti-DLG antibodies (D). Scale bars, 20 µm (A and B); and 30 µm (C and D).
Similar articles
- The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure.
Lahey T, Gorczyca M, Jia XX, Budnik V. Lahey T, et al. Neuron. 1994 Oct;13(4):823-35. doi: 10.1016/0896-6273(94)90249-6. Neuron. 1994. PMID: 7946331 Free PMC article. - The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse.
Guan B, Hartmann B, Kho YH, Gorczyca M, Budnik V. Guan B, et al. Curr Biol. 1996 Jun 1;6(6):695-706. doi: 10.1016/s0960-9822(09)00451-5. Curr Biol. 1996. PMID: 8793296 Free PMC article. - PAR-1 kinase phosphorylates Dlg and regulates its postsynaptic targeting at the Drosophila neuromuscular junction.
Zhang Y, Guo H, Kwan H, Wang JW, Kosek J, Lu B. Zhang Y, et al. Neuron. 2007 Jan 18;53(2):201-15. doi: 10.1016/j.neuron.2006.12.016. Neuron. 2007. PMID: 17224403 Free PMC article. - The drosophila neuromuscular junction: a model system for studying synaptic development and function.
Keshishian H, Broadie K, Chiba A, Bate M. Keshishian H, et al. Annu Rev Neurosci. 1996;19:545-75. doi: 10.1146/annurev.ne.19.030196.002553. Annu Rev Neurosci. 1996. PMID: 8833454 Review. - Invaginating Presynaptic Terminals in Neuromuscular Junctions, Photoreceptor Terminals, and Other Synapses of Animals.
Petralia RS, Wang YX, Mattson MP, Yao PJ. Petralia RS, et al. Neuromolecular Med. 2017 Sep;19(2-3):193-240. doi: 10.1007/s12017-017-8445-y. Epub 2017 Jun 13. Neuromolecular Med. 2017. PMID: 28612182 Free PMC article. Review.
Cited by
- Temporal coherency between receptor expression, neural activity and AP-1-dependent transcription regulates Drosophila motoneuron dendrite development.
Vonhoff F, Kuehn C, Blumenstock S, Sanyal S, Duch C. Vonhoff F, et al. Development. 2013 Feb 1;140(3):606-16. doi: 10.1242/dev.089235. Development. 2013. PMID: 23293292 Free PMC article. - Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-β/BMP signaling and orphan nuclear receptors.
Boulanger A, Farge M, Ramanoudjame C, Wharton K, Dura JM. Boulanger A, et al. PLoS One. 2012;7(7):e40255. doi: 10.1371/journal.pone.0040255. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792255 Free PMC article. - Immunofluorescence and image analysis pipeline for Drosophila motor neurons.
Brown JR, Phongthachit C, Sulkowski MJ. Brown JR, et al. Biol Methods Protoc. 2019;4(1):bpz010. doi: 10.1093/biomethods/bpz010. Epub 2019 Aug 1. Biol Methods Protoc. 2019. PMID: 31403085 Free PMC article. - Lepidopteran DALP, and its mammalian ortholog HIC-5, function as negative regulators of muscle differentiation.
Hu Y, Cascone PJ, Cheng L, Sun D, Nambu JR, Schwartz LM. Hu Y, et al. Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):10218-23. doi: 10.1073/pnas.96.18.10218. Proc Natl Acad Sci U S A. 1999. PMID: 10468589 Free PMC article. - Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein.
Thomas U, Ebitsch S, Gorczyca M, Koh YH, Hough CD, Woods D, Gundelfinger ED, Budnik V. Thomas U, et al. Curr Biol. 2000 Sep 21;10(18):1108-17. doi: 10.1016/s0960-9822(00)00696-5. Curr Biol. 2000. PMID: 10996791 Free PMC article.
References
- Apel ED, Merlie JP. The assembly of the postsynaptic apparatus. Curr. Opin. Neurobiol. 1995;5:62–67. - PubMed
- Atwood HL, Kwan I. Synaptic development in the crayfish opener muscle. J. Neurobiol. 1976;7:289–312. - PubMed
- Atwood HL, Nguyen PV. Neural adaptation incrayfish. Am. Zool. 1995;35:28–36.
- Atwood H, Govind CK, Wu C-F. Differential ultra-structure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J. Neurobiol. 1993;24:1008–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS030072/NS/NINDS NIH HHS/United States
- R21 NS070032/NS/NINDS NIH HHS/United States
- K04 NS01786/NS/NINDS NIH HHS/United States
- R01 NS70032/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases