Inhibition of granulocytic differentiation by mNotch1 - PubMed (original) (raw)
Inhibition of granulocytic differentiation by mNotch1
L A Milner et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Effective hematopoiesis requires the commitment of pluripotent and multipotent stem cells to distinct differentiation pathways, proliferation and maturation of cells in the various lineages, and preservation of pluripotent progenitors to provide continuous renewal of mature blood cells. While the importance of positive and negative cytokines in regulating proliferation and maturation of hematopoietic cells has been well documented, the factors and molecular processes involved in lineage commitment and self-renewal of multipotent progenitors have not yet been defined. In other developmental systems, cellular interactions mediated by members of the Notch gene family have been shown to influence cell fate determination by multipotent progenitors. We previously described the expression of the human Notch1 homolog, TAN-1, in immature hematopoietic precursors. We now demonstrate that constitutive expression of the activated intracellular domain of mouse Notch1 in 32D myeloid progenitors inhibits granulocytic differentiation and permits expansion of undifferentiated cells, findings consistent with the known function of Notch in other systems.
Figures
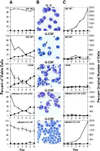
Figure 1
Diagram of the mNotch1 gene product and retroviral constructs. The molecule consists of an extracellular domain containing 36 tandem epidermal growth factor (EGF) repeats and 3 lin-12/Notch (LNR) repeats; a single transmembrane domain (TM); and an intracellular domain containing 6 cdc10/SWI6/ankyrin repeats, putative nuclear localization signals (NLS) and a C-terminal OPA/PEST region. The activated mNotch1 construct (MT-mNotch-ICΔOP) consists of the region of the intracellular domain including the cdc10 repeats and 3′ nuclear localization signals. The constructs included 5′ myc epitope tags (MT) to facilitate detection of protein expression. The control construct MT-mNotch1-ICΔOPΔCDC lacks the 5′ end of mNotch through the cdc10 repeats, but otherwise corresponds to MT-mNotch1-ICΔOP. Constructs were cloned into the LXSN retroviral vector that had been modified to contain a _Cla_I restriction site.
Figure 2
Effect of activated mNotch1 expression on the differentiation and proliferation of 32D cells. The parental 32D line (WT) and 32D cells transduced with the pLXSN retrovirus, pLXSN containing the active region of the intracellular domain of mNotch1 (mNotch1-ICΔOP), or a pLXSN-mNotch1 control lacking the cdc10 repeats (mNotch1-ICΔOPΔCDC) were evaluated for growth and differentiation in response to G-CSF. This figure represents one of four experiments, all having comparable results. (A) The relative percentages of viable cells remaining undifferentiated (○) or showing morphologic characteristics of mature granulocytes (▪) after successive days in culture. Plots for the retroviral lines represent the averages of four (LXSN) or six (mNotch1-ICΔOP and mNotch1-ICΔOPΔCDC) individual clones. Error bars = SEM for each set of clones; error bars are not included on the WT graphs since these represent a single population. (B) Representative Wright-stained cells. IL-3, undifferentiated parental 32D (WT) cells maintained in medium containing 10% WCM as a source of IL-3; G-CSF, 32D WT cells and representative clones expressing pLXSN, pLXSN-mNotch1-ICΔOPΔCDC or pLXSN-mNotch1-ICΔOP after 6 days in medium containing 1% WCM and 10 ng/ml G-CSF. The cells depicted in the second and fourth panels (32D WT and mNotch1-ICΔOPΔCDC) represent a single field condensed by deleting vacant areas to show a greater number of cells. The white and black arrows indicate band forms and segmented neutrophils, respectively. (C) The proliferation of undifferentiated cells (○) and mature granulocytes (▪) after successive days in culture with IL-3 (32D WT, Upper) or G-CSF. The total number of undifferentiated cells and mature granulocytes relative to the original number of cells plated are compared for the 32D parent line (WT) and each set of retroviral clones. Again the graphs represent the averages of four (LXSN) or six (mNotch1-ICΔOPΔCDC and mNotch1-ICΔOP) clones. SEMs were small; error bars are not depicted for the sake of clarity and would not change the impression created by the figure. Cells showing some characteristics of differentiation, but which were less mature than band cells were not included in either the undifferentiated or mature categories.
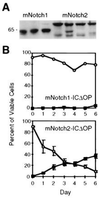
Figure 3
Comparison of mNotch1- and mNotch2-ICΔOP expression and effect on granulocytic differentiation of 32D cells. (A) Western blot of three representative mNotch1-ICΔOP and four mNotch2-ICΔOP 32D clones. Each lane contained a total cell lysate representing 5 × 106 cells electrophoresed through a 10% acrylamide gel, transferred to nitrocellulose, and immunostained using the 9e10 (α-myc) monoclonal antibody. The clones shown include those used for the experiment depicted in_B_. (B) G-CSF-induced granulocytic differentiation. In this experiment, three individual clones expressing mNotch2-ICΔOP, a representative mNotch1-ICΔOP clone, four LXSN-MT control clones, and the parental 32D WT cells were evaluated for differentiation in response to 50 ng/ml G-CSF and plotted as the relative percentages of viable cells remaining undifferentiated (○) or showing morphologic characteristics of mature granulocytes (▪). Differentiation of the mNotch2 clones was comparable to the 32D WT and LXSN-MT controls (not shown, but comparable to Fig. 2_A_).
Similar articles
- Notch signalling via RBP-J promotes myeloid differentiation.
Schroeder T, Just U. Schroeder T, et al. EMBO J. 2000 Jun 1;19(11):2558-68. doi: 10.1093/emboj/19.11.2558. EMBO J. 2000. PMID: 10835354 Free PMC article. - Notch signaling induces multilineage myeloid differentiation and up-regulates PU.1 expression.
Schroeder T, Kohlhof H, Rieber N, Just U. Schroeder T, et al. J Immunol. 2003 Jun 1;170(11):5538-48. doi: 10.4049/jimmunol.170.11.5538. J Immunol. 2003. PMID: 12759431 - Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling.
Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, Pear WS, Bernstein ID. Varnum-Finney B, et al. Nat Med. 2000 Nov;6(11):1278-81. doi: 10.1038/81390. Nat Med. 2000. PMID: 11062542 - Regulation of granulopoiesis by transcription factors and cytokine signals.
Ward AC, Loeb DM, Soede-Bobok AA, Touw IP, Friedman AD. Ward AC, et al. Leukemia. 2000 Jun;14(6):973-90. doi: 10.1038/sj.leu.2401808. Leukemia. 2000. PMID: 10865962 Review. - Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation.
Milner LA, Bigas A. Milner LA, et al. Blood. 1999 Apr 15;93(8):2431-48. Blood. 1999. PMID: 10194420 Review. No abstract available.
Cited by
- Neurobeachin regulates hematopoietic progenitor differentiation and survival by modulating Notch activity.
Ganuza M, Morales-Hernández A, Van Huizen A, Chabot A, Hall T, Caprio C, Finkelstein D, Kilimann MW, McKinney-Freeman S. Ganuza M, et al. Blood Adv. 2024 Aug 13;8(15):4129-4143. doi: 10.1182/bloodadvances.2023012426. Blood Adv. 2024. PMID: 38905595 Free PMC article. - Notch Signaling in Acute Inflammation and Sepsis.
Gallenstein N, Tichy L, Weigand MA, Schenz J. Gallenstein N, et al. Int J Mol Sci. 2023 Feb 9;24(4):3458. doi: 10.3390/ijms24043458. Int J Mol Sci. 2023. PMID: 36834869 Free PMC article. Review. - The Role of Notch and Wnt Signaling in MSC Communication in Normal and Leukemic Bone Marrow Niche.
Takam Kamga P, Bazzoni R, Dal Collo G, Cassaro A, Tanasi I, Russignan A, Tecchio C, Krampera M. Takam Kamga P, et al. Front Cell Dev Biol. 2021 Jan 8;8:599276. doi: 10.3389/fcell.2020.599276. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33490067 Free PMC article. Review. - Notch1 activation enhances proliferation via activation of cdc2 and delays differentiation of myeloid progenitors.
Nakamura M, Wu L, Griffin JD, Kojika S, Goi K, Inukai T, Sugita K. Nakamura M, et al. Leuk Res. 2018 Sep;72:34-44. doi: 10.1016/j.leukres.2018.07.022. Epub 2018 Jul 30. Leuk Res. 2018. PMID: 30086426 Free PMC article. - Mesenchymal stem cell-mediated Notch2 activation overcomes radiation-induced injury of the hematopoietic system.
Kim A, Shim S, Kim MJ, Myung JK, Park S. Kim A, et al. Sci Rep. 2018 Jun 18;8(1):9277. doi: 10.1038/s41598-018-27666-w. Sci Rep. 2018. PMID: 29915190 Free PMC article.
References
- Artavanis-Tsakonas S, Simpson P. Trends Genet. 1991;7:403–408. - PubMed
- Greenwald I, Rubin G M. Cell. 1992;68:271–281. - PubMed
- Fortini M E, Artavanis-Tsakonas S. Cell. 1993;75:1245–1247. - PubMed
- Muskavitch M A T. Dev Biol. 1994;166:415–430. - PubMed
- Artavanis-Tsakonas S, Matsuno K, Fortini M E. Science. 1995;268:225–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous