Disrupted splenic architecture, but normal lymph node development in mice expressing a soluble lymphotoxin-beta receptor-IgG1 fusion protein - PubMed (original) (raw)
Disrupted splenic architecture, but normal lymph node development in mice expressing a soluble lymphotoxin-beta receptor-IgG1 fusion protein
R Ettinger et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Early in ontogeny, the secondary lymphoid organs become populated with numerous cells of mesodermal origin which forms both the lymphoid and stromal elements. The critical receptor/ligand interactions necessary for lymphoid organogenesis to occur are for the most part unknown. Although lymphotoxin-alpha (LT alpha) has been shown to be required for normal lymph node, Peyer's patch, and splenic development, it is unclear if soluble LT alpha 3, and/or cell-bound lymphotoxin-alpha beta (LT alpha beta) mediate these developmental events. Here we report that blocking LT alpha beta/lymphotoxin-beta receptor (LT beta R) interaction in vivo by generating mice which express a soluble LT beta R-Fc fusion protein driven by the human cytomegalovirus promoter results in an array of anatomic abnormalities affecting both the spleen and Peyer's patches, but not the lymph nodes. These results demonstrate that surface LT alpha beta ligand plays a critical role in normal lymphoid organ development.
Figures
Figure 1
Reduced size of spleen and Peyer’s patches in mice expressing a soluble LTβR–Fc transgene. Three- to 15-week-old mice expressing high levels of LTβR–Fc transgene product in the sera (0.5–6.5 μg/ml) from two separate founder lines were weighed and sacrificed, and the weight of the thymi and spleens was determined. Small intestine Peyer’s patches were counted, and the serosal surface area was determined. The percent control was determined by directly comparing sex-matched nontransgenic littermates to transgene-positive mice. Horizontal lines represent the group averages, and the mean ± the SD are as follows: body weight, 91% ± 11.0 (40 mice from 10 separate litters); spleen weight, 75% ± 15.7 (35 mice from 9 separate litters); thymus weight, 103% ± 35.2 (34 mice from 8 separate litters); and Peyer’s patch area, 39% ± 23.9 (23 mice from 6 separate litters).
Figure 2
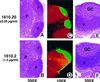
Normal architecture of lymph nodes and Peyer’s patches from LTβR–Fc-expressing mice. Tissue sections of lymph node (A_–_D) and Peyer’s patch (E and F) from either low-expressing (A, C, and E), or high-expressing (B, D, and_F_) 6-week-old female LTβR–Fc transgenic mice (1610.2 and 1610.20 from Table 1). Tissue sections were stained with either hematoxylin/eosin (A and B,E and F) (×200), or with antibodies specific for T-cell (anti-CD4, red) and B-cell (anti-B220, green) antigens (C and D) (×100). Yellow staining indicates colocalization of both T and B cells. F, P, and GC indicate the location of the B-cell-rich follicles, the T-cell-rich paracortex, and the germinal centers, respectively.
Figure 3
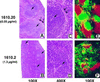
Blockade of LTαβ/LTβR interactions results in abnormal splenic architecture. Splenic tissue sections from mice expressing either low (A, C, and_E_) or high (B, D, and_F_) levels of the LTβR–Fc transgene, as described in Fig. 2. Formalin-fixed sections were stained with hematoxylin/eosin (A_–_D). A single arrow represents the location of the central arterioles, and a double arrow defines the marginal zones. Frozen tissue sections were stained with antibodies specific for T-cell (red) and B-cell (green) antigens (E and F), as described in Fig. 2. (A,B, E, and F, ×100;C and D, ×400.)
Figure 4
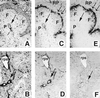
Absence of splenic marginal zones in mice expressing a soluble LTβR–Fc chimeric fusion protein. Frozen tissue sections of spleen from 10-week-old female mice expressing high levels of the LTβR–Fc protein (2.5 μg/ml, from founder 201) (B, D, and F) or transgenic-negative mice (A, C, and_E_) were stained with antibodies specific for reticular fibroblasts with ER-TR7 (A and B), marginal zone macrophages with ER-TR9 (C and_D_), or marginal zone metallophilic macrophages with MOMA-1 (E and F), as described. A single arrow represents the location of the central arterioles, and a double arrow defines the marginal zones. F, P, RP, and rps shows the location of the B-cell-rich follicles, the T-cell-rich PALS, the red pulp, and the red pulp sinus, respectively. (×125.)
Similar articles
- Effects of tumor necrosis factor and lymphotoxin on peripheral lymphoid tissue development.
Ettinger R, Mebius R, Browning JL, Michie SA, van Tuijl S, Kraal G, van Ewijk W, McDevitt HO. Ettinger R, et al. Int Immunol. 1998 Jun;10(6):727-41. doi: 10.1093/intimm/10.6.727. Int Immunol. 1998. PMID: 9678753 - Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor.
Pasparakis M, Alexopoulou L, Grell M, Pfizenmaier K, Bluethmann H, Kollias G. Pasparakis M, et al. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6319-23. doi: 10.1073/pnas.94.12.6319. Proc Natl Acad Sci U S A. 1997. PMID: 9177215 Free PMC article. - Transgenic expression of lymphotoxin restores lymph nodes to lymphotoxin-alpha-deficient mice.
Sacca R, Turley S, Soong L, Mellman I, Ruddle NH. Sacca R, et al. J Immunol. 1997 Nov 1;159(9):4252-60. J Immunol. 1997. PMID: 9379020 - Peyer's patch organogenesis--cytokines rule, OK?
Mayrhofer G. Mayrhofer G. Gut. 1997 Nov;41(5):707-9. doi: 10.1136/gut.41.5.707. Gut. 1997. PMID: 9414984 Free PMC article. Review. - Cytokine regulation of secondary lymphoid organ development.
Chaplin DD, Fu Y. Chaplin DD, et al. Curr Opin Immunol. 1998 Jun;10(3):289-97. doi: 10.1016/s0952-7915(98)80167-2. Curr Opin Immunol. 1998. PMID: 9638365 Review.
Cited by
- Multiple roles for tumor necrosis factor-alpha and lymphotoxin alpha/beta in immunity and autoimmunity.
McDevitt H, Munson S, Ettinger R, Wu A. McDevitt H, et al. Arthritis Res. 2002;4 Suppl 3(Suppl 3):S141-52. doi: 10.1186/ar570. Epub 2002 May 9. Arthritis Res. 2002. PMID: 12110133 Free PMC article. Review. - The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus.
Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. Steele C, et al. PLoS Pathog. 2005 Dec;1(4):e42. doi: 10.1371/journal.ppat.0010042. Epub 2005 Dec 9. PLoS Pathog. 2005. PMID: 16344862 Free PMC article. - Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen.
Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Ngo VN, et al. J Exp Med. 1999 Jan 18;189(2):403-12. doi: 10.1084/jem.189.2.403. J Exp Med. 1999. PMID: 9892622 Free PMC article. - Lymphotoxin-beta receptor signaling complex: role of tumor necrosis factor receptor-associated factor 3 recruitment in cell death and activation of nuclear factor kappaB.
VanArsdale TL, VanArsdale SL, Force WR, Walter BN, Mosialos G, Kieff E, Reed JC, Ware CF. VanArsdale TL, et al. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2460-5. doi: 10.1073/pnas.94.6.2460. Proc Natl Acad Sci U S A. 1997. PMID: 9122217 Free PMC article. - New insights into the cell biology of the marginal zone of the spleen.
Kraal G, Mebius R. Kraal G, et al. Int Rev Cytol. 2006;250:175-215. doi: 10.1016/S0074-7696(06)50005-1. Int Rev Cytol. 2006. PMID: 16861066 Free PMC article. Review.
References
- Ware C F, VanArsdale T L, Crowe P D, Browning J L. Curr Top Microbiol Immunol. 1995;198:175–218. - PubMed
- Tartaglia L A, Goeddel D V. Immunol Today. 1992;13:151–153. - PubMed
- Browning J L, Ngam-ek A, Lawton P, Demarinis J, Tizard R, Chow E P, Hession C, O’Brine-Greco B, Foley S F, Ware C F. Cell. 1993;72:847–856. - PubMed
- Crowe P D, VanArsdale T L, Walter B N, Ware C F, Hession C, Ehrenfels B, Browning J L, Din W S, Goodwin R G, Smith C A. Science. 1994;264:707–710. - PubMed
- De Togni P, Goellner J, Ruddle N H, Streeter P R, Fick A, Mariathasan S, Smith S C, Carlson R, Shornick L P, Strauss-Schoenberger J, Russell J H, Karr R, Chaplin D D. Science. 1994;264:703–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous