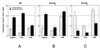Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin - PubMed (original) (raw)
Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin
G Schiavo et al. Proc Natl Acad Sci U S A. 1996.
Erratum in
- Proc Natl Acad Sci U S A 1997 Feb 4;94(3):1047
Abstract
The synaptic vesicle membrane protein synaptotagmin (tagmin) is essential for fast, calcium-dependent, neurotransmitter release and is likely to be the calcium sensor for exocytosis, because of its many calcium-dependent properties. Polyphosphoinositides are needed for exocytosis, but it has not been known why. We now provide a possible connection between these observations with the finding that the C2B domain of tagmin I binds phosphatidylinositol-4,5-bisphosphate (PIns-4,5-P2), its isomer phosphatidylinositol-3,4-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate (PIns-3,4,5-P3). Calcium ions switch the specificity of this binding from PIns-3,4,5-P3 (at calcium concentrations found in resting nerve terminals) to PIns-4,5-P2 (at concentration of calcium required for transmitter release). Inositol polyphosphates, known blockers of neurotransmitter release, inhibit the binding of both PIns-4,5-P2 and PIns-3,4,5-P3 to tagmin. Our findings imply that tagmin may operate as a bimodal calcium sensor, switching bound lipids during exocytosis. This connection to polyphosphoinositides, compounds whose levels are physiologically regulated, could be important for long-term memory and learning.
Figures
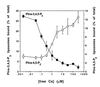
Figure 2
Calcium dependency of PIns-4,5-P2 and PIns-3,4,5-P3 binding to tagmin. GST-tagmin beads were simultaneously incubated with two populations of PC liposomes either containing PIns-4,5-P2 or PIns-3,4,5-P3 at variable Ca2+ concentrations (○, PIns-4,5-P2; ▪, PIns-3,4,5-P3). Liposome binding to tagmin and its domains was determined as described in Fig. 1.
Figure 4
The binding of PIns-4,5-P2 to recombinant and native tagmin is saturable and is competed by InsP6. (A) GST-tagmin (•) or GST alone (○) was incubated at increasing concentration of radioactive PIns-4,5-P2 in detergent (OG) micelles in the presence of 2 mM EGTA. Samples were analyzed as described in Fig. 1. (A) Tagmin/PIns-4,5-P2 molar ratio as function of the micellar PIns-4,5-P2 concentration. Dashed line represents the PIns-4,5-P2 specifically associated with tagmin. Parallel experiments using Triton X-100 [0.02% (wt/vol)] gave the same results (not shown). (B) GST-tagmin was incubated in the presence of saturable amount of PIns-4,5-P2 (6 μM) with increasing amount of InsP6 in the presence of 2 mM EGTA. (C) Immunoprecipitated native tagmin (•) or antitagmin antibody alone (○) were incubated with radioactive PIns-4,5-P2 in detergent micelles in the presence of 2 mM EGTA. Dashed line represents the PIns-4,5-P2 specifically associated with tagmin. (D) SDS/PAGE profile of the immunopurified native tagmin used in C. In addition to the tagmin monomer with an apparent molecular weight of 65 kDa, an SDS-resistant tagmin dimer is also visible (37).
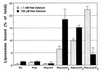
Figure 1
Tagmin binds specifically to polyphosphoinositide-containing liposomes in a calcium-dependent manner. GST-tagmin was incubated with liposomes containing pure PC or PC together with distinct polyphosphoinositides (as indicated) in the absence (shaded bars, 2 mM EGTA) or presence of calcium ions (solid bars, 100 μM free Ca2+). Lipid binding was quantified by liquid scintillation counting of the radioactive PC used as tracer and expressed as percent of total radioactivity used. Specific binding was calculated by subtracting the nonspecific lipid interaction of GST (2.4 ± 0.7%) from individual samples. Similar results were obtained both with small and large unilamellar vesicles, thus suggesting that size and curvature of the liposome do not influence the binding.
Figure 3
The polyphosphoinositides binding site on tagmin is localized to the C2B domain. GST fusion proteins containing tagmin or its N-terminal (C2A; aa 96–265; 10 μg) or the C-terminal (C2B; aa 248–421; 10 μg) domains were incubated with liposomes containing PC and 25% (wt/wt) PS (A), 1% (wt/wt) PIns-4,5-P2 (B), or 1% (wt/wt) PIns-3,4,5-P3 (C) (shaded bars, 2 mM EGTA; solid bars, 100 μM free Ca2+). Liposome binding to tagmin and its domains was determined as described in Fig. 1.
Similar articles
- Allosteric stabilization of calcium and phosphoinositide dual binding engages several synaptotagmins in fast exocytosis.
Kobbersmed JRL, Berns MMM, Ditlevsen S, Sørensen JB, Walter AM. Kobbersmed JRL, et al. Elife. 2022 Aug 5;11:e74810. doi: 10.7554/eLife.74810. Elife. 2022. PMID: 35929728 Free PMC article. - Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I.
Sheng ZH, Yokoyama CT, Catterall WA. Sheng ZH, et al. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5405-10. doi: 10.1073/pnas.94.10.5405. Proc Natl Acad Sci U S A. 1997. PMID: 9144250 Free PMC article. - Role of the C2B domain of synaptotagmin in vesicular release and recycling as determined by specific antibody injection into the squid giant synapse preterminal.
Fukuda M, Moreira JE, Lewis FM, Sugimori M, Niinobe M, Mikoshiba K, Llinás R. Fukuda M, et al. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10708-12. doi: 10.1073/pnas.92.23.10708. Proc Natl Acad Sci U S A. 1995. PMID: 7479869 Free PMC article. - Synaptotagmin: a calcium-sensitive inhibitor of exocytosis?
Popov SV, Poo MM. Popov SV, et al. Cell. 1993 Jul 2;73(7):1247-9. doi: 10.1016/0092-8674(93)90352-q. Cell. 1993. PMID: 8100739 Review. No abstract available. - Synaptic vesicle fusion and synaptotagmin: 2B or not 2B?
O'Connor V, Lee AG. O'Connor V, et al. Nat Neurosci. 2002 Sep;5(9):823-4. doi: 10.1038/nn0902-823. Nat Neurosci. 2002. PMID: 12196805 Review. No abstract available.
Cited by
- Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits.
Gaidarov I, Keen JH. Gaidarov I, et al. J Cell Biol. 1999 Aug 23;146(4):755-64. doi: 10.1083/jcb.146.4.755. J Cell Biol. 1999. PMID: 10459011 Free PMC article. - Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion.
Mayer A, Scheglmann D, Dove S, Glatz A, Wickner W, Haas A. Mayer A, et al. Mol Biol Cell. 2000 Mar;11(3):807-17. doi: 10.1091/mbc.11.3.807. Mol Biol Cell. 2000. PMID: 10712501 Free PMC article. - Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells.
Tucker WC, Edwardson JM, Bai J, Kim HJ, Martin TF, Chapman ER. Tucker WC, et al. J Cell Biol. 2003 Jul 21;162(2):199-209. doi: 10.1083/jcb.200302060. Epub 2003 Jul 14. J Cell Biol. 2003. PMID: 12860971 Free PMC article. - Structural insights into the Ca2+ and PI(4,5)P2 binding modes of the C2 domains of rabphilin 3A and synaptotagmin 1.
Guillén J, Ferrer-Orta C, Buxaderas M, Pérez-Sánchez D, Guerrero-Valero M, Luengo-Gil G, Pous J, Guerra P, Gómez-Fernández JC, Verdaguer N, Corbalán-García S. Guillén J, et al. Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20503-8. doi: 10.1073/pnas.1316179110. Epub 2013 Dec 3. Proc Natl Acad Sci U S A. 2013. PMID: 24302762 Free PMC article. - The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells.
Evans JH, Gerber SH, Murray D, Leslie CC. Evans JH, et al. Mol Biol Cell. 2004 Jan;15(1):371-83. doi: 10.1091/mbc.e03-05-0338. Epub 2003 Sep 17. Mol Biol Cell. 2004. PMID: 13679516 Free PMC article.
References
- Rothman J E. Nature (London) 1994;372:55–63. - PubMed
- Rothman J E, Wieland F T. Science. 1996;272:227–234. - PubMed
- Südhof T C. Nature (London) 1995;375:645–653. - PubMed
- Littleton J T, Bellen H J. Trends Neurosci. 1995;18:177–183. - PubMed
- Newton A C. Curr Biol. 1995;5:973–976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources