Detection of ligands in regions anatomically connected to neurons expressing the Eph receptor Bsk: potential roles in neuron-target interaction - PubMed (original) (raw)
Detection of ligands in regions anatomically connected to neurons expressing the Eph receptor Bsk: potential roles in neuron-target interaction
J H Zhang et al. J Neurosci. 1996.
Abstract
Neuron-target interaction is a key feature in the establishment of neuronal networks. However, the underlying mechanism remains unclear. We have shown that at the time of target innervation, Bsk, an eph family receptor, is expressed at high levels in several brain regions including the hippocampus, olfactory bulb, and retina. To study whether the ligands are expressed in the target tissues, we investigated the expression of Bsk ligands using a ligand-affinity probe, Bsk-AP, which consisted of the extracellular domain of Bsk fused in frame with a human placental alkaline phosphatase. These analyses showed that the ligands were expressed at high levels in the developing septum, hypothalamus, olfactory neural epithelium, and tectum. In situ hybridization studies revealed that at least three different factors were responsible for the Bsk-AP binding. In the septum, Elf-1, Lerk3 (Eff-2), and AL-1/Lerk7 were transcribed. In the hypothalamus, AL-1/Lerk7 was the ligand detected by Bsk-AP. In the olfactory system, high levels of Lerk3 were detected in the sensory neurons. Both Elf-1 and AL-1/Lerk7 were present in the tectum. These ligand-positive areas are known to be anatomically connected to Bsk-expressing regions. These observations strongly suggest that Bsk and the ligands participate in neuron-target interactions in multiple systems and provide support for their involvement in topographic projection.
Figures
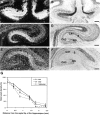
Fig. 1.
Differential Bsk expression along the mediolateral hippocampal axis. Bsk mRNA levels detected by _in situ_hybridization at different positions along the mediolateral axis are shown. A, B, Dark- and bright-field photomicrographs of a medial (septal) hippocampal section.C, D, Dark- and bright-field photomicrographs of a section at an intermediate mediolateral level.E, F, Dark- and bright-field pictures of a lateral (temporal) hippocampal section. Slides shown here and in the following figures were counterstained with thionin. G, Quantitative analysis of Bsk in situ hybridization signals at different mediolateral hippocampal levels. S, Subiculum; DG, dentate gyrus. Scale bars, 200 μm.
Fig. 2.
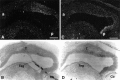
Expression of Bsk ligand(s) in the adult septum.A, E, Bsk-AP binding activity in the septum.B, F, Elf-1 expression. C,G, Lerk3 expression. D, H, AL-1/Lerk7 expression. A_–_D, Coronal sections. E_–_H, Higher magnifications of the septal regions, showing that the expression of the ligands was restricted to the ventral lateral septum. ac, Anterior commissure; cc, corpus callosum; f, fornix; BST, bed nucleus of the stria terminalis;Cpu, caudate putamen; Ctx, cerebral cortex; LS, lateral septum; MS, medial septum; Se, septum; VDB, vertical diagonal band; Scale bars: A, 1.2 mm; E, 0.6 mm.
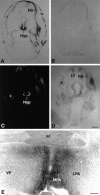
Fig. 3.
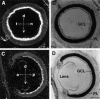
Expression of Bsk ligand in the hypothalamus.A, B, Coronal sections of E15 mouse embryos were stained with Bsk-AP (A) or control alkaline phosphatase (B). C, D, Dark- and bright-field photomicrographs of E15 mouse embryos hybridized to AL-1/Lerk7 cRNA probe. E, Coronal section through the preoptic area of an adult mouse brain stained with Bsk-AP. In situ hybridization of similar sections with AL-1/Lerk7 cRNA probe gave identical pattern of mRNA expression. Ac, Anterior commissure; E, eye; FB, primordium of frontal bone; Hyp, hypothalamus;LV, lateral ventricle; LPA, lateral preoptic area; MPA, medial preoptic area;VP, ventral pallidum. Scale bars: D, 0.7 mm; E, 0.2 mm.
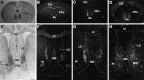
Fig. 4.
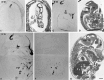
Complementary expression of Bsk and ligand(s) in the E18 olfactory system. A, Photomicrograph of a sagittal section of the olfactory bulb and nasal epithelium stained with Bsk-AP (areas indicated by arrows).B, Bsk receptor expression detected by in situ hybridization in a similar section as in A.AOB, Accessory olfactory bulb; MOB, main olfactory bulb; NE, nasal epithelium; _ON_olfactory nerve. Scale bar, 0.5 mm.
Fig. 5.
Differential distribution of Bsk and Lerk3 in the mouse olfactory system. A, Bsk-AP staining of a coronal section through the adult olfactory bulb. B, Detection of Bsk receptor by in situ hybridization in the adult olfactory bulb. Note the complementary patterns of ligand and receptor expression. C, A higher magnification of Bsk-AP-stained adult glomerular structures, showing that ligand-positive (arrowhead) and ligand-negative glomeruli (arrow, circled). D, Higher magnification of Bsk in situ hybridization signals in the adult olfactory bulb, showing that the expression in the mitral and granular cells is not uniform. Numerous Bsk-negative cells were clearly visible (arrows). The average silver grain density (% area of cells covered) of the Bsk-positive cells was 11.3 ± 1.6 compared to 2 ± 0.27 for the Bsk-negative cells and 1.9 ± 0.17 for the background level. E,F, Low and high magnification of Lerk3 in situ hybridization signals in P3 olfactory nasal epithelium, showing an uneven distribution. GBCL, Globose basal cell layer; Gr, granule cell layer; Mi, mitral cell layer; NC, nasal cavity; NE, nasal epithelium; ON, olfactory nerve; ORNL, olfactory receptor neuron layer; SCL, sustentacular cell layer. Scale bars: A, B, 1.76 mm;C, 100 μm; E, 200 μm;D, F, 25 μm.
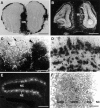
Fig. 6.
Bsk expression in the retina. A,B, Dark- and bright-field photomicrographs of a coronal view of a E18 mouse retina. C, D, Dark- and bright-field photomicrographs of a sagittal view of a E18 mouse retina. The signal intensity in A and _C_are not comparable because the results were obtained in separate experiments. a, Anterior; p, posterior;d, dorsal; v, ventral; t, temporal; n, nasal; GCL, ganglion cell layer; PL, plexiform layer. Scale bar, 320 μm.
Fig. 7.
Sagittal views of expression of Bsk ligand(s) during mouse embryogenesis. Neighboring sagittal sections from E10 (A, B), E13 (C,D), and E15 (E_–_G) embryos were stained with Bsk-AP (A, C,E) or H&E (B, D,G). Human placental alkaline phosphatase not fused to Bsk was used in parallel sections as a control (F). In E10 and E13 embryos, no specific staining was observed in control sections. In E15 (F) and E18 embryos, endogenous heat-resistant alkaline phosphatase activity was detected only in the intestine. die, Diencephalon;mes, mesencephalon; met, metencephalon;mye, myelencephalon; tel, telencephalon;t, tongue; Hyp, hypothalamus;SC, spinal cord; T, tectum; Scale bars:B, 1 mm; D, G, 2 mm.
Fig. 8.
Expression of Elf-1 and AL-1/Lerk7 in E16 tectum.A, B, Dark- and bright-field photomicrographs of a parasagittal section through the tectum hybridized with Elf-1 probe. C, D, Dark- and bright-field views of a serial section to A and_B_, hybridized with a AL-1/Lerk7 probe. a, Anterior tectum; p, posterior tectum; Cb, cerebellum; T, tectum; Teg, tegmentum. Scale bar: 250 μm.
Fig. 9.
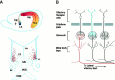
Proposed models for the function of Bsk and its ligands in the hippocamposeptal and olfactory systems.A, The top panel shows that the ligands located in the ventral lateral septum serve to restrict the medial hippocampal neurons (Bsk-positive, red) from innervating this region (ligand-positive, blue), which is topographically inappropriate for the medial neurons. However, the ligands allow the innervation of the ventral lateral septum by the lateral hippocampal neurons, because they lack the receptor Bsk (yellow). The bottom panel shows that three different ligands, Elf-1 (purple), Lerk3 (orange), and AL-1/Lerk7 (light blue) in combination specify a dorsomedial (DM)-to-ventrolateral (VL) gradient that may serve as spatial code for hippocamposeptal topographic mapping. B, Bsk and its ligand may act to specify different types of synapses between the odor receptor neurons and the mitral or tufted cells. Because the interaction of Eph family ligands and receptors results in inhibition of axonal outgrowth, no synapse may be formed between ligand-positive (blue) odor receptors and Bsk-positive (red) mitral or tufted cells. Thus, only three different types of synapses, ligand-positive odor receptor neurons to Bsk-negative mitral cells (black), ligand-negative odor receptor neurons (black) to Bsk-positive mitral cells, and ligand-negative odor receptor neurons to Bsk-negative mitral cells, are possible. ac, Anterior commissure; DM, dorsomedial region of the lateral septum; HDB, horizontal limb of diagonal band; Hip, hippocampus;Lig, ligand gradient; LS, lateral septum;MS, medial septum; VDB, vertical limb of diagonal band; VL, ventrolateral region of the lateral septum.
Similar articles
- Regulation of topographic projection by the Eph family receptor Bsk (EphA5) and its ligands.
Zhou R. Zhou R. Cell Tissue Res. 1997 Nov;290(2):251-9. doi: 10.1007/s004410050929. Cell Tissue Res. 1997. PMID: 9321686 Review. - Dynamic expression suggests multiple roles of the eph family receptor brain-specific kinase (Bsk) during mouse neurogenesis.
Zhang JH, Pimenta AF, Levitt P, Zhou R. Zhang JH, et al. Brain Res Mol Brain Res. 1997 Jul;47(1-2):202-14. doi: 10.1016/s0169-328x(97)00051-x. Brain Res Mol Brain Res. 1997. PMID: 9221918 - Marsupial retinocollicular system shows differential expression of messenger RNA encoding EphA receptors and their ligands during development.
Vidovic M, Marotte LR, Mark RF. Vidovic M, et al. J Neurosci Res. 1999 Jul 15;57(2):244-54. doi: 10.1002/(SICI)1097-4547(19990715)57:2<244::AID-JNR10>3.0.CO;2-D. J Neurosci Res. 1999. PMID: 10398302 - Ephrin-A binding and EphA receptor expression delineate the matrix compartment of the striatum.
Janis LS, Cassidy RM, Kromer LF. Janis LS, et al. J Neurosci. 1999 Jun 15;19(12):4962-71. doi: 10.1523/JNEUROSCI.19-12-04962.1999. J Neurosci. 1999. PMID: 10366629 Free PMC article. - [Molecular mechanisms underlying the formation of the topographic retinotectal map].
Yuasa-Kawada J, Noda M. Yuasa-Kawada J, et al. Tanpakushitsu Kakusan Koso. 2000 Feb;45(3 Suppl):307-15. Tanpakushitsu Kakusan Koso. 2000. PMID: 10707635 Review. Japanese. No abstract available.
Cited by
- New model of retinocollicular mapping predicts the mechanisms of axonal competition and explains the role of reverse molecular signaling during development.
Grimbert F, Cang J. Grimbert F, et al. J Neurosci. 2012 Jul 11;32(28):9755-68. doi: 10.1523/JNEUROSCI.6180-11.2012. J Neurosci. 2012. PMID: 22787061 Free PMC article. - Molecular mechanisms of optic axon guidance.
Inatani M. Inatani M. Naturwissenschaften. 2005 Dec;92(12):549-61. doi: 10.1007/s00114-005-0042-5. Epub 2005 Oct 12. Naturwissenschaften. 2005. PMID: 16220285 Review. - Loss-of-function analysis of EphA receptors in retinotectal mapping.
Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Feldheim DA, et al. J Neurosci. 2004 Mar 10;24(10):2542-50. doi: 10.1523/JNEUROSCI.0239-03.2004. J Neurosci. 2004. PMID: 15014130 Free PMC article. - Formation of persistent hyperplastic primary vitreous in ephrin-A5-/- mice.
Son AI, Sheleg M, Cooper MA, Sun Y, Kleiman NJ, Zhou R. Son AI, et al. Invest Ophthalmol Vis Sci. 2014 Mar 19;55(3):1594-606. doi: 10.1167/iovs.13-12706. Invest Ophthalmol Vis Sci. 2014. PMID: 24550361 Free PMC article. - Further analysis of the lens of ephrin-A5-/- mice: development of postnatal defects.
Son AI, Cooper MA, Sheleg M, Sun Y, Kleiman NJ, Zhou R. Son AI, et al. Mol Vis. 2013;19:254-66. Epub 2013 Feb 3. Mol Vis. 2013. PMID: 23401654 Free PMC article.
References
- Bartley TD, Hunt RW, Welcher AA, Boyle WJ, Parker VP, Lindberg RA, Lu HS, Colombero AM, Elliott RL, Guthrie BA, Holst PL, Skrine JD, Toso RJ, Zhang M, Fernandez E, Trail G, Varnum B, Yarden Y, Hunter T, Fox GM. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–560. - PubMed
- Boxberg YV, Deiss S, Schwarz U. Guidance and topographic stablization of nasal chick retinal axons on target-derived components in vitro. Neuron. 1993;10:345–357. - PubMed
- Brambilla R, Klein R. Telling axons where to grow: a role for Eph receptor tyrosine kinases in guidance. Mol Cell Neurosci. 1996;6:487–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous