Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson's disease - PubMed (original) (raw)
Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson's disease
C Winkler et al. J Neurosci. 1996.
Abstract
Glial cell line-derived neurotrophic factor (GDNF) has been shown to exert neuroprotective effects on dopamine (DA) neurons in vivo. Here we report long-term rescue of nigral DA neurons after delayed short-term GDNF administration in a rat lesion model that reproduces the slowly progressing degenerative process seen in Parkinson's disease. GDNF injected close to the substantia nigra provided near-complete protection and persistent survival of the lesioned nigral neurons for at least 4 months after discontinuation of GDNF treatment. Long-term rescue of the nigral cells, however, was not accompanied by any significant reinnervation of the lesioned striatal target or any signs of functional recovery in either drug-induced or spontaneous motor behaviors. We conclude that not only preservation of the nigral DA neurons but also restoration of striatal DA function is necessary for functional recovery in the rat Parkinson model.
Figures
Fig. 1.
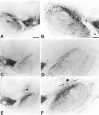
Transient GDNF treatment protects nigral DA neurons from lesion-induced cell death. Coronal sections through the SN and the VTA separated by the MTN (arrowheads).A, B, The side contralateral to the intrastriatal 6-OHDA lesion. Most of the TH-IR cells are located in the SN pars compacta (SNc), whereas a few TH-IR cells and TH-IR fibers can be seen in the SN pars reticulata (SNr). C, D, The midbrain ipsilateral to the 6-OHDA lesion in a vehicle-injected animal. Few TH-IR cells remain in the SN, and there is a marked loss of TH-IR fibers in the SNr. No obvious effect of the intrastriatal 6-OHDA lesion can be seen on TH-IR cells in the VTA. E,F, The side ipsilateral to the 6-OHDA lesion in a representative animal that had received transient GDNF treatment. Many TH-IR cell profiles can be seen in the SNc, and the TH-IR fiber density in the SNr is preserved at normal levels. The injection site is located dorsal to the SNc (asterisk), and apparent sprouting of TH-positive fibers from the SN toward the injection site can be seen (arrows). Scale bars: A, 500 μm;B, 250 μm.
Fig. 2.
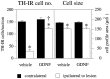
Nigral DA neurons are rescued by transient GDNF treatment but remain in an atrophic state. TH-IR cells in the SN were counted in sections that showed a clear separation of SN and VTA by the MTN (see Fig. 1). This landmark was seen in three sections per series. Five months after the intrastriatal 6-OHDA lesion and 4 months after cessation of either vehicle or GDNF injections, the number of TH-IR cells in the ipsilateral SN was reduced to 19.4 ± 4% compared with the contralateral side in vehicle-injected animals, whereas 77.8 ± 4.1% of the cells were spared in GDNF-injected animals.Left panel, Asterisks indicate significant difference from the contralateral side (p < 0.001, paired t test);dagger, significant difference from vehicle-injected group (p < 0.001, ANOVA with post hoc Scheffé test). In both groups, the surviving TH-positive cells were significantly reduced in size compared with the contralateral side (right panel,asterisks, p < 0.001, paired_t_ test), and there was no difference between the groups, thus indicating that TH-positive neurons rescued by transient GDNF treatment remained in an atrophic state. Error bars represent SEM.
Fig. 3.
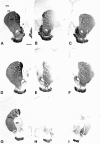
Variability of the striatal 6-OHDA lesion. The lesion-induced loss of striatal TH-IR fiber density varied among the animals, and two extreme cases are illustrated in this figure.A, D, G, Three sections illustrating the intact control side (contralateral to the lesion) at the level of the lesion site (D) and rostral (A) or caudal (G) to the lesion site. The levels shown in this figure refer to the measurement of TH-IR fiber density as shown in Figure 4. CC, Corpus callosum;CPu, caudate–putamen; Ctx, cortex;GP, globus pallidus; NA, nucleus accumbens; OT, olfactory tubercle; S, septum. B, E, H, Corresponding sections from the lesioned striatum of a vehicle-injected animal displaying an extensive loss of TH-IR fiber density in the head and tail of the caudate–putamen. Although the loss of TH-IR is almost complete at the site of the 6-OHDA injection (E) and caudal to it (H), some TH-IR fibers are spared in the medial and the ventrolateral part of the rostral striatum (B). The densitometric analysis of this specimen indicated a 89% reduction in TH-IR fiber density at the level of the lesion site. C, F, I, Lesioned striatum from a vehicle-injected animal displaying a smaller lesion that is largely confined to the dorsolateral part of the caudate–putamen (F, I). Whereas many fibers are spared rostrally from the injection site (C), there is a clear overall reduction of TH-IR fiber density, as seen by the intensity of the staining. The densitometric analysis revealed a 38% reduction in TH-staining intensity at the level of the lesion site in this animal. Scale bar, 1 mm.
Fig. 4.
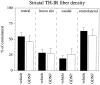
GDNF administration over the SN cell bodies fails to preserve DA nerve terminals in the striatum. Striatal TH-IR fiber density, measured as mean optical density (expressed in percent of the contralateral nonlesioned side) is shown at different rostrocaudal levels in relation to the site of 6-OHDA injection. TH-IR fiber density is significantly different from the contralateral side in both groups at all measured levels (p < 0.001, paired_t_ test).
Fig. 5.
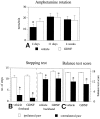
Performance in motor initiation and akinesia tests is impaired in the 6-OHDA-lesioned animals. A, Ipsilateral rotational net asymmetry scores induced by amphetamine (5 mg/kg, i.p.), expressed as full-body turns per minute over 90 min. Time points given indicate days after the intrastriatal 6-OHDA lesioning. The high rotational scores seen at 4 d after lesioning, i.e., the day before the first vehicle or GDNF injection, indicate extensive damage of the striatal DA nerve terminals at the time when GDNF treatment was initiated (¤ p < 0.05, ANOVA with_post hoc_ Scheffé test). Initiation of forelimb side-stepping movements (B) and the ability of the rats to initiate forelimb movements to regain balance (C) are impaired on the side contralateral to the striatal 6-OHDA lesion. GDNF treatment has no effect on the performance in these tasks. The numbers given are the means of six tests, performed at 4 months after the 6-OHDA lesioning. Asterisks, Significant difference between the paws, paired t test. Error bars represent SEM.
Fig. 7.
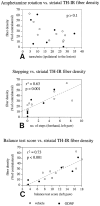
Performance in motor initiation tasks but not in amphetamine-induced rotation is correlated with striatal TH-IR fiber density. A, Amphetamine-induced rotation at 4 weeks after lesioning of the single groups or of both groups pooled together shows no correlation with striatal TH-IR fiber density (p > 0.05, _r_2 = 0.2, both groups combined). Performance of the animals in the stepping test (B) and the balance test (C) is significantly correlated with striatal TH-IR fiber density (both for the single experimental groups and for the two groups combined), as recorded either at the level of the injection site (shown here) or at the levels rostral and caudal to the injection site (data not shown).
Fig. 6.
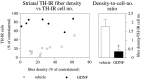
Correlation between striatal TH-IR fiber density and TH-IR nigral cell number. In the vehicle-injected animals, the striatal TH-IR fiber density (recorded at the level of the lesion site) is significantly correlated to the number of surviving TH-IR cells in the SN (p < 0.001;_r_2 = 0.7; _open circles_), whereas there is no correlation in GDNF-injected animals (_p_ > 0.8;_r_2 = 0, filled circles). Similar correlations also were obtained for TH-IR fiber densities recorded rostral or caudal to the lesion site. As nigral cell bodies but not striatal nerve terminals are rescued by supranigral GDNF treatment, the ratio between TH-IR fiber density and TH-IR cell number is drastically reduced. Dagger, Significant difference from the vehicle-injected group (p < 0.001, ANOVA with post hoc Scheffé test). Error bars represent SEM.
Similar articles
- Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson's disease after administration into the striatum or the lateral ventricle.
Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Björklund A. Rosenblad C, et al. Eur J Neurosci. 1999 May;11(5):1554-66. doi: 10.1046/j.1460-9568.1999.00566.x. Eur J Neurosci. 1999. PMID: 10215908 - Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.
Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Björklund A, et al. Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review. - Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson's disease.
Björklund A, Rosenblad C, Winkler C, Kirik D. Björklund A, et al. Neurobiol Dis. 1997;4(3-4):186-200. doi: 10.1006/nbdi.1997.0151. Neurobiol Dis. 1997. PMID: 9361295 Review.
Cited by
- GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson's disease.
d'Anglemont de Tassigny X, Pascual A, López-Barneo J. d'Anglemont de Tassigny X, et al. Front Neuroanat. 2015 Feb 13;9:10. doi: 10.3389/fnana.2015.00010. eCollection 2015. Front Neuroanat. 2015. PMID: 25762899 Free PMC article. Review. - Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson's disease.
Voutilainen MH, Bäck S, Pörsti E, Toppinen L, Lindgren L, Lindholm P, Peränen J, Saarma M, Tuominen RK. Voutilainen MH, et al. J Neurosci. 2009 Jul 29;29(30):9651-9. doi: 10.1523/JNEUROSCI.0833-09.2009. J Neurosci. 2009. PMID: 19641128 Free PMC article. - GDNF protection against 6-OHDA-induced reductions in potassium-evoked overflow of striatal dopamine.
Cass WA, Manning MW. Cass WA, et al. J Neurosci. 1999 Feb 15;19(4):1416-23. doi: 10.1523/JNEUROSCI.19-04-01416.1999. J Neurosci. 1999. PMID: 9952418 Free PMC article. - Restorative plasticity of dopamine neuronal transplants depends on the degree of hemispheric dominance.
Nikkhah G, Falkenstein G, Rosenthal C. Nikkhah G, et al. J Neurosci. 2001 Aug 15;21(16):6252-63. doi: 10.1523/JNEUROSCI.21-16-06252.2001. J Neurosci. 2001. PMID: 11487648 Free PMC article. - Mechanistic Insight from Preclinical Models of Parkinson's Disease Could Help Redirect Clinical Trial Efforts in GDNF Therapy.
Delgado-Minjares KM, Martinez-Fong D, Martínez-Dávila IA, Bañuelos C, Gutierrez-Castillo ME, Blanco-Alvarez VM, Cardenas-Aguayo MD, Luna-Muñoz J, Pacheco-Herrero M, Soto-Rojas LO. Delgado-Minjares KM, et al. Int J Mol Sci. 2021 Oct 28;22(21):11702. doi: 10.3390/ijms222111702. Int J Mol Sci. 2021. PMID: 34769132 Free PMC article. Review.
References
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. - PubMed
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AE. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol. 1995;355:479–489. - PubMed
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. - PubMed
- Dunnett SB, Björklund A, Schmidt RH, Stenevi U, Iversen SD. Intracerebral grafting of neuronal cell suspensions. IV. Behavioural recovery in rats with unilateral 6-OHDA lesions following implantation of nigral cell suspensions in different forebrain sites. Acta Physiol Scand. 1983;522:29–37. - PubMed
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional specificity. Brain. 1991;114:2283–2301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources