Neural mechanisms for visual memory and their role in attention - PubMed (original) (raw)
Review
Neural mechanisms for visual memory and their role in attention
R Desimone. Proc Natl Acad Sci U S A. 1996.
Abstract
Recent studies show that neuronal mechanisms for learning and memory both dynamically modulate and permanently alter the representations of visual stimuli in the adult monkey cortex. Three commonly observed neuronal effects in memory-demanding tasks are repetition suppression, enhancement, and delay activity. In repetition suppression, repeated experience with the same visual stimulus leads to both short- and long-term suppression of neuronal responses in subpopulations of visual neurons. Enhancement works in an opposite fashion, in that neuronal responses are enhanced for objects with learned behavioral relevance. Delay activity is found in tasks in which animals are required to actively hold specific information "on-line" for short periods. Repetition suppression appears to be an intrinsic property of visual cortical areas such as inferior temporal cortex and is thought to be important for perceptual learning and priming. By contrast, enhancement and delay activity may depend on feedback to temporal cortex from prefrontal cortex and are thought to be important for working memory. All of these mnemonic effects on neuronal responses bias the competitive interactions that take place between stimulus representations in the cortex when there is more than one stimulus in the visual field. As a result, memory will often determine the winner of these competitions and, thus, will determine which stimulus is attended.
Figures
Figure 1
Five ways in which neuronal activity is modified during the formation or expression of memory traces. (Adapted from ref. .)
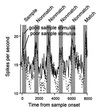
Figure 2
Response histograms averaged from a population of 40 prefrontal neurons that had significant sample-selective delay activity. Responses are shown separately for trials in which a preferred or “good” stimulus was used as the sample and trials in which a nonpreferred or “poor” stimulus was used as the sample (bin width, 40 ms). (Adapted from ref. .)
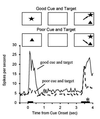
Figure 3
Response histograms averaged from a population of 22 IT neurons recorded while the monkey performed a visual search task. A cue stimulus was briefly presented at the start of the trial, followed by a blank delay period during which the animal maintained fixation at the center of the display. At the end of the delay, a choice array containing two stimuli at random locations was presented and the animal was rewarded from making a saccadic eye movement to the stimulus that matched the cue (target). The presentation periods for the cue and array are indicated by horizontal bars. The cues were chosen for each cell such that one would activate a cell when presented alone (“good” cue) and one would only poorly activate the cell when presented alone (“poor” cue). Responses to identical choice arrays are shown separately for trials with the good versus poor stimulus for the recorded cells used as the cue. When the good cue was used, activity was higher during the delay period, and responses to the choice array remained high. When the poor cue was used, activity was lower during the delay period, and responses to the identical choice array were suppressed approximately 200 ms after the onset of the array. Thus, only cells selective for the target stimulus remained active, all other cells being suppressed. The saccadic eye movement to the target (asterisk) began about 300 ms after the onset of the array, well after responses to the nontarget stimuli were suppressed. (Adapted from refs. and .)
Similar articles
- Responses of neurons in inferior temporal cortex during memory-guided visual search.
Chelazzi L, Duncan J, Miller EK, Desimone R. Chelazzi L, et al. J Neurophysiol. 1998 Dec;80(6):2918-40. doi: 10.1152/jn.1998.80.6.2918. J Neurophysiol. 1998. PMID: 9862896 - Selective attention modulates neural substrates of repetition priming and "implicit" visual memory: suppressions and enhancements revealed by FMRI.
Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J. Vuilleumier P, et al. J Cogn Neurosci. 2005 Aug;17(8):1245-60. doi: 10.1162/0898929055002409. J Cogn Neurosci. 2005. PMID: 16197681 Clinical Trial. - Mnemonic Encoding and Cortical Organization in Parietal and Prefrontal Cortices.
Masse NY, Hodnefield JM, Freedman DJ. Masse NY, et al. J Neurosci. 2017 Jun 21;37(25):6098-6112. doi: 10.1523/JNEUROSCI.3903-16.2017. Epub 2017 May 24. J Neurosci. 2017. PMID: 28539423 Free PMC article. - Functional brain imaging studies of cortical mechanisms for memory.
Ungerleider LG. Ungerleider LG. Science. 1995 Nov 3;270(5237):769-75. doi: 10.1126/science.270.5237.769. Science. 1995. PMID: 7481764 Review. - Monkey perirhinal cortex is critical for visual memory, but not for visual perception: reexamination of the behavioural evidence from monkeys.
Hampton RR. Hampton RR. Q J Exp Psychol B. 2005 Jul-Oct;58(3-4):283-99. doi: 10.1080/02724990444000195. Q J Exp Psychol B. 2005. PMID: 16194970 Review.
Cited by
- Distributed representation of visual objects by single neurons in the human brain.
Valdez AB, Papesh MH, Treiman DM, Smith KA, Goldinger SD, Steinmetz PN. Valdez AB, et al. J Neurosci. 2015 Apr 1;35(13):5180-6. doi: 10.1523/JNEUROSCI.1958-14.2015. J Neurosci. 2015. PMID: 25834044 Free PMC article. - Divisive Normalization Predicts Adaptation-Induced Response Changes in Macaque Inferior Temporal Cortex.
Kaliukhovich DA, Vogels R. Kaliukhovich DA, et al. J Neurosci. 2016 Jun 1;36(22):6116-28. doi: 10.1523/JNEUROSCI.2011-15.2016. J Neurosci. 2016. PMID: 27251630 Free PMC article. - Dissociable neural mechanisms underlie currently-relevant, future-relevant, and discarded working memory representations.
Lorenc ES, Vandenbroucke ARE, Nee DE, de Lange FP, D'Esposito M. Lorenc ES, et al. Sci Rep. 2020 Jul 8;10(1):11195. doi: 10.1038/s41598-020-67634-x. Sci Rep. 2020. PMID: 32641712 Free PMC article. - Mirror neurons in monkey area F5 do not adapt to the observation of repeated actions.
Caggiano V, Pomper JK, Fleischer F, Fogassi L, Giese M, Thier P. Caggiano V, et al. Nat Commun. 2013;4:1433. doi: 10.1038/ncomms2419. Nat Commun. 2013. PMID: 23385578 - Neural correlates of multisensory perceptual learning.
Powers AR 3rd, Hevey MA, Wallace MT. Powers AR 3rd, et al. J Neurosci. 2012 May 2;32(18):6263-74. doi: 10.1523/JNEUROSCI.6138-11.2012. J Neurosci. 2012. PMID: 22553032 Free PMC article.
References
- Desimone R. Science. 1992;258:245–246. - PubMed
- Desimone R, Ungerleider L G. In: Neural Mechanisms of Visual Processing in Monkeys. Boller F, Grafman J, editors. Vol. 14. New York: Elsevier; 1989. pp. 267–299.
- Ungerleider L G, Mishkin M. In: Two Cortical Visual Systems. Ingle D, Goodale M A, Mansfield R J W, editors. Cambridge, MA: MIT Press; 1982. pp. 549–586.
- Ungerleider L G, Haxby J V. Curr Opin Neurobiol. 1994;4:157–165. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials