Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry - PubMed (original) (raw)
Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry
B Gillo et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Capacitative Ca2+ entry is a component of the inositol-lipid signaling in which depletion of inositol 1,4,5-trisphosphate (InsP3)-sensitive Ca2+ stores activates Ca2+ influx by a mechanism that is still unknown. This pathway plays a central role in cellular signaling, which is mediated by many hormones, neurotransmitters, and growth factors. Studies of Drosophila photoreceptors provided the first putative capacitative Ca2+ entry mutant designated transient receptor potential (trp) and a Drosophila gene encoding TRP-like protein (trpl). It is not clear how the Ca2+ store depletion signal is relayed to the plasma membrane and whether both TRP and TRPL participate in this process. We report here that coexpressing Drosophila TRP and TRPL in Xenopus oocytes synergistically enhances the endogenous Ca(2+)-activated Cl- current and produces a divalent inward current. Both of these currents are activated by Ca2+ store depletion. In the absence of Ca2+, Mg2+ is the main charge carrier of the divalent current. This current is characterized by lanthanum sensitivity and a voltage-dependent blocking effect of Mg2+, which is relieved at both hyperpolarizing (inward rectification) and depolarizing (outward rectification) potentials. The store-operated divalent current is neither observed in native oocytes nor in oocytes expressing either TRP or TRPL alone. The production of this current implicates a cooperative action of TRP and TRPL in the depletion-activated current.
Figures
Figure 1
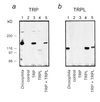
Heterologous expression of TRP and TRPL gives rise to large amounts of TRP and TRPL proteins. Western blots show the expression of TRP (a) and TRPL (b) in oocytes injected with cRNA of trp, trpl, and trp+trpl, as indicated. Control (uninjected oocytes) and extract of three wild-type Drosophila heads (Drosophila) show that the equivalent molecular sizes of the Drosophila TRP and TRPL were produced in the oocytes, which cannot be confused with endogenous oocyte protein of similar structure and size. The data of this figure were highly reproducible in oocytes from different frogs (n = 8).
Figure 3
Functional coexpression of TRP+TRPL in_Xenopus_ oocytes produced a capacitative Ca2+ entry system revealed by ICl,Ca and IdSOC. Shown is a single experiment, employing several oocytes from a single frog that were maintained and treated together. (a) Measurements of currents (ICl,Ca) in thapsigargin-treated oocytes (1 μM in Ca2+-free solution for 1.5–2 hr). ICl,Ca was activated by stepping the holding voltage from −10 mV to −30 mV (upper traces) and to −120 mV (bottom traces) to show the relatively small instantaneous leak current in solution containing 1 mM Ca2+ (see Fig. 2). Oocytes were injected with cRNA 5 days before the measurements. (b) Measurements of IdSOC were carried out as described in_a_. The same oocytes were perfused with Ca2+-free ND96 solution (10 mM Mg2+). In some of the measurements, 2 mM EGTA was injected into the oocytes 1–2 hr before the recordings, but no significant effect on IdSOC was found. IdSOC was totally and reversibly blocked by addition of 1 mM La3+ to the perfusate (c and d). IdSOC was also blocked reversibly by 500 μM, but not by 50 μM, La3+ (n = 12). (c and d) Histograms summarizing the results from all the oocytes of the same experimental run of_a_ and b. Five to 10 oocytes were used for each of the experimental groups of a and_b_. The histograms present the mean and SEM of the peak ICl,Ca (c) and maximal IdSOC (d) measured at −120-mV holding potentials after the instantaneous leak currents were subtracted from all current traces. The TRP+TRPL group was significantly different from the other oocyte groups of c (P < 0.01). The control and TRP groups were not significantly different (_P_ > 0.05).
Figure 5
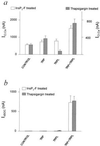
Histograms summarizing ICl,Ca and IdSOC in TRP, TRPL, and TRP+TRPL-expressing oocytes in experiments similar to those in Fig. 3. (a) ICl,Ca measured in InsP3-F-treated oocytes (left ordinate) or thapsigargin-treated oocytes (right ordinate). Eleven independent experiments used 16–64 oocytes in each group. The TRP+TRPL groups were significantly different from the other oocyte groups (P < 0.01), whereas the other oocyte groups were not significantly different in the InsP3-F-treated oocytes (_P_ > 0.05). In the thapsigargin-treated oocytes all groups were significantly different from each other (P < 0.01 for a comparison between the control TRPL and TRP+TRPL groups; P < 0.05 for a comparison between the control and TRP group). (b) IdSOC measured in InsP3-F or thapsigargin-treated oocytes. Seven independent experiments used 8–22 oocytes in each group.
Figure 2
Coexpression of TRP and TRPL largely enhances Ca2+ influx into Ca2+ stores-depleted oocytes. (a) Confocal images of ratio changes between resting and peak Ca2+ levels during application of Ca2+ containing solution (2 mM). Changes of ratios were coded as the green-to-red gradient together with the_z_-axis magnitude. One pair of optical sections across the oocyte of about 70 μm deep was analyzed to form each image. The “ring” shape of the image is due to the melanin pigmentation, which interferes with the fluorescence detection from the center of the oocyte. (b) A plot of changes in fluorescence ratio as a function of time in the oocytes shown in a. The ordinate plots the average ratio difference (R̄_o(640/530) −_R̄_P(640/530)) where_R̄ is the averaged fluorescence ratio of the scan before (_R̄_o) or during and after (_R̄_P) Ca2+ application. The ratio of the pixels was averaged for a whole scan after threshold noise reduction. The normalized average ratio difference of TRP+TRPL was 2.08 ± 0.23 (n = 5) times the averaged control (n = 9), as compared with TRPL alone at 0.82 ± 0.10 (n = 5) times the control. The TRP+TRPL group was significantly different from the other oocyte groups (P < 0.01), whereas the other oocyte groups were not significantly different (_P_ > 0.05). The initial Ca2+ level was variable among oocytes; therefore, ratio differences were used to demonstrate consistent results similar to those shown in Fig. 3 b and c. The magnitude of the ratio differences varied in different experiments; therefore, the summary results were normalized. The time of Ca2+ application and removal is indicated by up- and down-pointing arrows, respectively.
Figure 2
Coexpression of TRP and TRPL largely enhances Ca2+ influx into Ca2+ stores-depleted oocytes. (a) Confocal images of ratio changes between resting and peak Ca2+ levels during application of Ca2+ containing solution (2 mM). Changes of ratios were coded as the green-to-red gradient together with the_z_-axis magnitude. One pair of optical sections across the oocyte of about 70 μm deep was analyzed to form each image. The “ring” shape of the image is due to the melanin pigmentation, which interferes with the fluorescence detection from the center of the oocyte. (b) A plot of changes in fluorescence ratio as a function of time in the oocytes shown in a. The ordinate plots the average ratio difference (R̄_o(640/530) −_R̄_P(640/530)) where_R̄ is the averaged fluorescence ratio of the scan before (_R̄_o) or during and after (_R̄_P) Ca2+ application. The ratio of the pixels was averaged for a whole scan after threshold noise reduction. The normalized average ratio difference of TRP+TRPL was 2.08 ± 0.23 (n = 5) times the averaged control (n = 9), as compared with TRPL alone at 0.82 ± 0.10 (n = 5) times the control. The TRP+TRPL group was significantly different from the other oocyte groups (P < 0.01), whereas the other oocyte groups were not significantly different (_P_ > 0.05). The initial Ca2+ level was variable among oocytes; therefore, ratio differences were used to demonstrate consistent results similar to those shown in Fig. 3 b and c. The magnitude of the ratio differences varied in different experiments; therefore, the summary results were normalized. The time of Ca2+ application and removal is indicated by up- and down-pointing arrows, respectively.
Figure 4
Current-voltage relationship of IdSOC in oocytes expressing TRP+TRPL. Inward and outward rectification typical of Drosophila light-activated current are shown. Current-voltage relationship (I-V curve) plotting the leak-subtracted maximal IdSOC as a function of holding voltage in InsP3-F (10 μM) or thapsigargin-treated oocytes incubated in Ca2+-free medium (see Fig. 3 a and_b_). Solutions are as in Fig. 3_b_. The I-V curves were measured before (•) and after (○) Na+ was replaced by_N_-methyl-
d
-glucamine (NMDG, 96 mM). Graphs show the average of currents obtained from nine oocytes of a single experiment. Very similar results were obtained in five other experiments. (Inset) Current-voltage relationship of the leak-subtracted peak LIC responses to identical 100-ms light flashes of orange light (OG 590, Schott edge filter) recorded from_Drosophila_ isolated ommatidia during whole-cell voltage clamp recordings as described (22).
Similar articles
- Role of Drosophila TRP in inositide-mediated Ca2+ entry.
Minke B, Selinger Z. Minke B, et al. Mol Neurobiol. 1996 Apr;12(2):163-80. doi: 10.1007/BF02740652. Mol Neurobiol. 1996. PMID: 8818149 Review. - Stimulation of Drosophila TrpL by capacitative Ca2+ entry.
Estacion M, Sinkins WG, Schilling WP. Estacion M, et al. Biochem J. 1999 Jul 1;341 ( Pt 1)(Pt 1):41-9. Biochem J. 1999. PMID: 10377243 Free PMC article. - [Molecular candidates for capacitative calcium entry channel].
Wang YH, Shao FY. Wang YH, et al. Sheng Li Ke Xue Jin Zhan. 2001 Oct;32(4):302-6. Sheng Li Ke Xue Jin Zhan. 2001. PMID: 12545855 Review. Chinese.
Cited by
- A novel capacitative calcium entry channel expressed in excitable cells.
Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalié A, Flockerzi V. Philipp S, et al. EMBO J. 1998 Aug 3;17(15):4274-82. doi: 10.1093/emboj/17.15.4274. EMBO J. 1998. PMID: 9687496 Free PMC article. - Mechanism of store-operated calcium entry.
Dutta D. Dutta D. J Biosci. 2000 Dec;25(4):397-404. doi: 10.1007/BF02703793. J Biosci. 2000. PMID: 11120592 Review. - Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres.
Kurebayashi N, Ogawa Y. Kurebayashi N, et al. J Physiol. 2001 May 15;533(Pt 1):185-99. doi: 10.1111/j.1469-7793.2001.0185b.x. J Physiol. 2001. PMID: 11351027 Free PMC article. - Mechanism and functional significance of TRPC channel multimerization.
Villereal ML. Villereal ML. Semin Cell Dev Biol. 2006 Dec;17(6):618-29. doi: 10.1016/j.semcdb.2006.10.010. Epub 2006 Nov 1. Semin Cell Dev Biol. 2006. PMID: 17158075 Free PMC article. Review. - Homo- and heteromeric assembly of TRP channel subunits.
Schaefer M. Schaefer M. Pflugers Arch. 2005 Oct;451(1):35-42. doi: 10.1007/s00424-005-1467-6. Epub 2005 Jun 22. Pflugers Arch. 2005. PMID: 15971080
References
- Putney J W J. Cell Calcium. 1990;11:611–624. - PubMed
- Hoth M, Penner R. Nature (London) 1992;355:353–356. - PubMed
- Zweifach A, Lewis R S. J Biol Chem. 1995;270:14445–14451. - PubMed
- Lewis R S, Cahalan M D. Annu Rev Immunol. 1995;13:623–653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous