Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution - PubMed (original) (raw)
Cultured melanocytes from dilute mutant mice exhibit dendritic morphology and altered melanosome distribution
D W Provance Jr et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Mutant alleles at the dilute unconventional myosin heavy chain locus cause diluted coat color, opisthotonic seizures, and death. The dilute coat color phenotype is caused by irregular clumping of pigment in the hair, but amounts of melanin are unchanged from wild-type controls. The melanocyte phenotype has been described as adendritic, since hair bulb and Harderian gland melanocytes appear to be rounded in tissue sections. These observations do not exclude the possibility that the processes lack pigment, since the melanocyte shape was judged by the distribution of melanin. We have tested this hypothesis by culturing primary melanocytes from dilute mutant and wild-type mice. The mutant melanocytes do not lack processes; instead, they exhibit a concentrated perinuclear distribution of melanosomes, while wild-type melanocytes have a very uniform cytoplasmic distribution of melanosomes. Electron micrographs show no detectable differences in melanosome morphology or maturation between dilute and wild-type melanocytes. Immunofluorescence experiments indicate that the dilute protein is concentrated in regions of the cytoplasm that contain melanosomes. These experiments show that the dilute myosin is necessary for the localization of melanosomes, either by active transport or tethering.
Figures
Figure 1
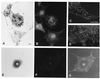
Phase-contrast micrograph of wild-type (A) and_d_v_/d_v (B) primary melanocytes, shown at the same magnification. The regions between the accumulated melanosomes in the_d_v_/d_v cells (B) contain cytoplasm lacking melanosomes. (Bar = 50 μm.)
Figure 2
Thin-section electron micrograph of_d_v_/d_v melanocyte showing the presence of Stage 2, 3, and 4 melanosomes. (Bar = 500 nm.)
Figure 3
Bright-field (showing melanosomal distribution) (A and E) and immunofluorescence (B–D, F, and G) photomicrographs of wild-type (A–D) and_d_v_/d_v (E–G) primary melanocytes. Antiserum to a dilute tail peptide was used as the primary antibody in all panels except_D_, for which an antibody to the carboxyl terminus of tyrosinase (αPEP7) was used. G is an underexposure of the same negative shown in F; it shows the background labeling and the extent of the cell’s cytoplasm. The cell at the middle of the left margin of B is a fibroblast that cannot be seen in A. (Bars: A, 10 μm;C, 5 μM. C and D have the same magnification; the remaining panels have the same magnification as A.) The difference between the punctate staining of the anti-dilute antibody and the ring-like staining of the melanosome periphery by αPEP7 (arrowheads) can be seen in at higher magnification in C and D.
Figure 4
Bright-field (showing melanosomal distribution) (A and D) and immunofluorescence (B–F) photomicrographs of dilute suppressor (a/a d_v/d_v dsu/dsu) (A–C) and leaden (C57BL/6J fz ln/ln) (D–F) primary melanocytes, using the anti-dilute tail peptide antiserum as primary antibody.C and F are underexposures of the same negatives shown in B and E, respectively, to show the extent of the cytoplasm of the cells. (Bar = 10 μm.)
Similar articles
- Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes.
Wu XS, Masedunskas A, Weigert R, Copeland NG, Jenkins NA, Hammer JA. Wu XS, et al. Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):E2101-9. doi: 10.1073/pnas.1209397109. Epub 2012 Jul 2. Proc Natl Acad Sci U S A. 2012. PMID: 22753477 Free PMC article. - The predominant defect in dilute melanocytes is in melanosome distribution and not cell shape, supporting a role for myosin V in melanosome transport.
Wei Q, Wu X, Hammer JA 3rd. Wei Q, et al. J Muscle Res Cell Motil. 1997 Oct;18(5):517-27. doi: 10.1023/a:1018659117569. J Muscle Res Cell Motil. 1997. PMID: 9350005 - Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo.
Wu X, Bowers B, Rao K, Wei Q, Hammer JA 3rd. Wu X, et al. J Cell Biol. 1998 Dec 28;143(7):1899-918. doi: 10.1083/jcb.143.7.1899. J Cell Biol. 1998. PMID: 9864363 Free PMC article. - Making sense of melanosome dynamics in mouse melanocytes.
Wu X, Hammer JA 3rd. Wu X, et al. Pigment Cell Res. 2000 Aug;13(4):241-7. doi: 10.1034/j.1600-0749.2000.130405.x. Pigment Cell Res. 2000. PMID: 10952391 Review. - The dilute locus and Griscelli syndrome: gateways towards a better understanding of melanosome transport.
Westbroek W, Lambert J, Naeyaert JM. Westbroek W, et al. Pigment Cell Res. 2001 Oct;14(5):320-7. doi: 10.1034/j.1600-0749.2001.140503.x. Pigment Cell Res. 2001. PMID: 11601653 Review.
Cited by
- The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles.
Elkind NB, Walch-Solimena C, Novick PJ. Elkind NB, et al. J Cell Biol. 2000 Apr 3;149(1):95-110. doi: 10.1083/jcb.149.1.95. J Cell Biol. 2000. PMID: 10747090 Free PMC article. - Tyrosinase Depletion Prevents the Maturation of Melanosomes in the Mouse Hair Follicle.
Paterson EK, Fielder TJ, MacGregor GR, Ito S, Wakamatsu K, Gillen DL, Eby V, Boissy RE, Ganesan AK. Paterson EK, et al. PLoS One. 2015 Nov 30;10(11):e0143702. doi: 10.1371/journal.pone.0143702. eCollection 2015. PLoS One. 2015. PMID: 26619124 Free PMC article. - Inhibition of dendrite formation in mouse melanocytes transiently transfected with antisense DNA to myosin Va.
Edgar AJ, Bennett JP. Edgar AJ, et al. J Anat. 1999 Aug;195 ( Pt 2)(Pt 2):173-84. doi: 10.1046/j.1469-7580.1999.19520173.x. J Anat. 1999. PMID: 10529054 Free PMC article. - Molecular genetic dissection of mouse unconventional myosin-VA: head region mutations.
Huang JD, Cope MJ, Mermall V, Strobel MC, Kendrick-Jones J, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. Huang JD, et al. Genetics. 1998 Apr;148(4):1951-61. doi: 10.1093/genetics/148.4.1951. Genetics. 1998. PMID: 9560408 Free PMC article. - Structural basis of cargo recognitions for class V myosins.
Wei Z, Liu X, Yu C, Zhang M. Wei Z, et al. Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):11314-9. doi: 10.1073/pnas.1306768110. Epub 2013 Jun 24. Proc Natl Acad Sci U S A. 2013. PMID: 23798443 Free PMC article.
References
- Silvers W K. The Coat Colors of Mice. New York: Springer; 1979.
- Searle A G. Heredity. 1952;6:395–401.
- Russell E S. Genetics. 1949;34:146–166. - PubMed
- Mooseker M S, Cheney R E. Annu Rev Cell Dev Biol. 1995;11:633–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases