Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus - PubMed (original) (raw)
Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus
Z Grossman et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Immature CD4+ CD8+ thymocytes expressing T-cell antigen receptors (TCR) are selected by TCR-mediated recognition of peptides associated with major histocompatibility complex molecules on thymic stromal cells. Selection ensures reactivity of the mature cells to foreign antigens and tolerance to self. Although much has been learned about the factors that determine whether a thymocyte with a given specificity will be positively or negatively selected, selection as an aspect of the developmental process as a whole is less well-understood. Here we invoke a model in which thymocytes tune their response characteristics individually and dynamically in the course of development. Cellular development and selection are driven by receptor-mediated metabolic perturbations. Perturbation is a measure of the net intracellular change induced by external stimulation. It results from the integration of several signals and countersignals over time and therefore depends on the environment and the maturation stage of the cell. Individual cell adaptation limits the range of perturbations. Such adaptation renders thymocytes less sensitive to the level of stimulation per se, but responsive to environmental changes in that level. This formulation begins to explain the mechanisms that link developmental and selection events to each other.
Figures
Figure 1
The TAT model. The kinetics of intracellular signal intensity (wavy line), activation threshold (upper smooth line), and viability-maintenance threshold (lower smooth line) are schematically illustrated. Five examples of different cells or of the same cell in different microenvironments are shown. (a) A cell undergoing stationary stimulation of a relatively small intensity. The cell maintains its viability without being fully activated. This is due to the signal intensity remaining below the activation threshold and regularly exceeding the viability-maintenance threshold. (b) A cell undergoing high-intensity stationary stimulation. Activation does not occur since the baseline and the associated thresholds are adjusted to the level of stimulation. (c) A gradual increase in the level of stimulation occurs, driving upward the baseline and the thresholds so that activation does not occur. Threshold tuning has allowed a smooth transition from the level of activity shown in a to that of b. (d) As in a and_b_, but an increase in the signal intensity occurs abruptly causing a strong perturbation (even though the peak intensity does not exceed intensities occurring in b). The activation threshold is surpassed, resulting in (full) activation. (e) As in a, but the intensity falls abruptly at some point (with the baseline adjusting more slowly), remaining below the viability-maintenance threshold for a sufficiently long time to cause death-by-neglect.
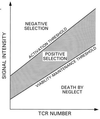
Figure 2
Threshold tuning during gradual increase in TCR expression by DP thymocytes. Illustrated are selection boundaries for the signal intensity, induced in a DP thymocyte, as a function of TCR number. For higher levels of receptor expression, the average signal intensity (i.e., the baseline activity level) is higher, entailing higher activation threshold and viability-maintenance threshold. The thymocyte is not activated as TCR number increases if the fluctuating signal intensity (not shown) does not exceed the activation threshold. At higher intensities, negative selection occurs. If the intensity falls below the viability-maintenance threshold for too long, death-by-neglect may result.
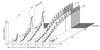
Figure 3
Typical signal intensity profiles of positively and negatively selected cells during thymic development. For positive selection, signal intensity should not exceed the activation threshold (upper curve) and should regularly exceed the viability-maintenance threshold (dotted line). The bottom line represents the baseline activity. Several examples are shown, ordered from right to left by increasing affinities for the selecting MHC–ligand combination. (a) Early death-by-neglect; (b and_c_) positive selection. (d) Negative selection as a result of a rapid increase in TCR number occurring toward the transition phase from the DP to the single-positive (SP) phenotype. (e) Cortical negative selection, occurring as the rate of increase in signal intensity is accelerated during the DP phase. (f) Negative selection of immature thymocytes upon transition from the double negative (DN) into the DP developmental state (possibly associated with an early receptor-aggregation event).
Similar articles
- Cross-talk between the T cell antigen receptor and the glucocorticoid receptor regulates thymocyte development.
Ashwell JD, King LB, Vacchio MS. Ashwell JD, et al. Stem Cells. 1996 Sep;14(5):490-500. doi: 10.1002/stem.140490. Stem Cells. 1996. PMID: 8888490 Review. - Subcellular distribution of Lck during CD4 T-cell maturation in the thymic medulla regulates the T-cell activation threshold.
Stephen TL, Wilson BS, Laufer TM. Stephen TL, et al. Proc Natl Acad Sci U S A. 2012 May 8;109(19):7415-20. doi: 10.1073/pnas.1119272109. Epub 2012 Apr 23. Proc Natl Acad Sci U S A. 2012. PMID: 22529380 Free PMC article. - T cell receptor-mediated recognition of self-ligand induces signaling in immature thymocytes before negative selection.
Abe R, Ishida Y, Yui K, Katsumata M, Chused TM. Abe R, et al. J Exp Med. 1992 Aug 1;176(2):459-68. doi: 10.1084/jem.176.2.459. J Exp Med. 1992. PMID: 1500856 Free PMC article. - Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene.
Strasser A, Harris AW, von Boehmer H, Cory S. Strasser A, et al. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1376-80. doi: 10.1073/pnas.91.4.1376. Proc Natl Acad Sci U S A. 1994. PMID: 8108419 Free PMC article. - Thymocyte selection: not by TCR alone.
Amsen D, Kruisbeek AM. Amsen D, et al. Immunol Rev. 1998 Oct;165:209-29. doi: 10.1111/j.1600-065x.1998.tb01241.x. Immunol Rev. 1998. PMID: 9850863 Review.
Cited by
- Defective selection of thymic regulatory T cells accompanies autoimmunity and pulmonary infiltrates in Tcra-deficient mice double transgenic for human La/Sjögren's syndrome-B and human La-specific TCR.
Yaciuk JC, Pan Y, Schwarz K, Pan ZJ, Maier-Moore JS, Kosanke SD, Lawrence C, Farris AD. Yaciuk JC, et al. J Immunol. 2015 Feb 15;194(4):1514-22. doi: 10.4049/jimmunol.1400319. Epub 2015 Jan 12. J Immunol. 2015. PMID: 25582858 Free PMC article. - Cooperative enhancement of specificity in a lattice of T cell receptors.
Chan C, George AJ, Stark J. Chan C, et al. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5758-63. doi: 10.1073/pnas.101113698. Proc Natl Acad Sci U S A. 2001. PMID: 11344310 Free PMC article. - Constant regulation for stable CD8 T-cell functional avidity and its possible implications for cancer immunotherapy.
Gilfillan CB, Hebeisen M, Rufer N, Speiser DE. Gilfillan CB, et al. Eur J Immunol. 2021 Jun;51(6):1348-1360. doi: 10.1002/eji.202049016. Epub 2021 Mar 30. Eur J Immunol. 2021. PMID: 33704770 Free PMC article. Review. - Releasing the Immune System Brakes Using siRNAs Enhances Cancer Immunotherapy.
Sioud M. Sioud M. Cancers (Basel). 2019 Feb 3;11(2):176. doi: 10.3390/cancers11020176. Cancers (Basel). 2019. PMID: 30717461 Free PMC article. Review. - Modeling T cell antigen discrimination based on feedback control of digital ERK responses.
Altan-Bonnet G, Germain RN. Altan-Bonnet G, et al. PLoS Biol. 2005 Nov;3(11):e356. doi: 10.1371/journal.pbio.0030356. Epub 2005 Oct 25. PLoS Biol. 2005. PMID: 16231973 Free PMC article.
References
- Jameson S C, Hogquist K A, Bevan M J. Annu Rev Immunol. 1995;13:93–126. - PubMed
- Kawai K, Ohashi P S. Nature (London) 1995;374:68–69. , and correction (1995) 378, 419. - PubMed
- Rothenberg E. Immunologist. 1995;3:172–175.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials