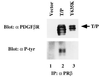The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways - PubMed (original) (raw)
The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways
M Carroll et al. Proc Natl Acad Sci U S A. 1996.
Abstract
The TEL/PDGF beta R fusion protein is the product of the t(5;12) translocation in patients with chronic myelomonocytic leukemia. The TEL/PDGF beta R is an unusual fusion of a putative transcription factor, TEL, to a receptor tyrosine kinase. The translocation fuses the amino terminus of TEL, containing the helix-loop-helix (HLH) domain, to the transmembrane and cytoplasmic domain of the PDGF beta R. We hypothesized that TEL/PDGF beta R self-association, mediated by the HLH domain of TEL, would lead to constitutive activation of the PDGF beta R tyrosine kinase domain and cellular transformation. Analysis of in vitro-translated TEL/ PDGF beta R confirmed that the protein self-associated and that self-association was abrogated by deletion of 51 aa within the TEL HLH domain. In vivo, TEL/PDGF beta R was detected as a 100-kDa protein that was constitutively phosphorylated on tyrosine and transformed the murine hematopoietic cell line Ba/F3 to interleukin 3 growth factor independence. Transformation of Ba/F3 cells required the HLH domain of TEL and the kinase activity of the PDGF beta R portion of the fusion protein. Immunoblotting demonstrated that TEL/PDGF beta R associated with multiple signaling molecules known to associate with the activated PDGF beta R, including phospholipase C gamma 1, SHP2, and phosphoinositol-3-kinase. TEL/PDGF beta R is a novel transforming protein that self-associates and activates PDGF beta R-dependent signaling pathways. Oligomerization of TEL/PDGF beta R that is dependent on the TEL HLH domain provides further evidence that the HLH domain, highly conserved among ETS family members, is a self-association motif.
Figures
Figure 1
Schematic representation of TEL, PDGFβR, T/P, and mutations of T/P.
Figure 2
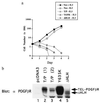
T/P transforms Ba/F3 cells to IL-3 factor independence. (a) Ba/F3 cells were transfected with pcDNA3, pcDNA3 T/P, the kinase-inactive mutant, Y635K, or the ΔHLH mutant and selected for G418 resistance. G418-resistant cells were selected for growth in the absence of IL-3. Neo-resistant (2 × 104 cells; pcDNA3, Y635K, and ΔHLH) or IL-3-independent cells (T/P) were washed free of IL-3 and plated on day 0 in RPMI 1640/10% FCS. Viable cells were counted on each day. (b) Expression of T/P and mutants in Ba/F3 cells. Transfected Ba/F3 cells were lysed in 1% Triton X-100/150 mM NaCl/50 mM Tris, pH 8.0, plus protease inhibitors. Lysates were separated by SDS/PAGE, transferred to nitrocellulose, and blotted with anti-PDGFβR antisera. Lanes 2 and 3 show two separate transfections with wild-type T/P.
Figure 3
T/P is constitutively tyrosine-phosphorylated in Ba/F3 cells. Ba/F3 cells were transfected with pcDNA3 alone (lane 1), T/P (lane 2), or a kinase-inactive mutant with a tyrosine-to-lysine mutation at the site corresponding to Y635 in the human PDGFβR (Y635K; lane 3). Cells were selected in 1 mg/ml of G418, and T/P cells were subsequently selected for growth in the absence of IL-3. Cells were lysed and immunoprecipitated with an antibody to the human PDGFβR (Upstate Biotechnology). Immunoprecipitates were separated by SDS/PAGE, transferred to nitrocellulose, and Western blotted using the indicated antisera and enhanced chemiluminescence (ECL) detection methods. All constructs direct the synthesis of a doublet due to the use of an alternative start site for translation in the TEL portion of the fusion cDNA.
Figure 4
T/P dimerizes in vitro and dimerization requires the HLH domain. cDNA constructs for the indicated mutations of T/P were cloned into the pcDNA3 expression vector, and_in vitro_ transcription/translation was performed according to the manufacturer’s instruction (Promega TNT kit) using radiolabeled [35S]methionine. Quantity of DNA added was adjusted to give approximately equal amounts of each translated protein. For lanes 7–12, one-half of the reaction mixture was removed and immunoprecipitated with an antisera to the carboxyl terminus of human PDGFβR. Total reaction products or total immunoprecipitates were separated by SDS/PAGE. The gel was fixed, treated with Enhance (Amersham), dried, and exposed to film at −70° for 30 min.
Figure 5
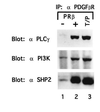
T/P associates with signaling molecules. Ba/F3–PDGFβR and Ba/F3 T/P cells were lysed and immunoprecipitated with anti-PDGFβR antisera. Immunoprecipitates were separated by SDS/PAGE, transferred to nitrocellulose, and blotted with the indicated antibodies. Ba/F3–PDGFβR were either deprived of IL-3 for 4 hr (−) or stimulated with 100 ng of recombinant human PDGF B for 10 min at 37° (+).
Similar articles
- Oligomerization of the ABL tyrosine kinase by the Ets protein TEL in human leukemia.
Golub TR, Goga A, Barker GF, Afar DE, McLaughlin J, Bohlander SK, Rowley JD, Witte ON, Gilliland DG. Golub TR, et al. Mol Cell Biol. 1996 Aug;16(8):4107-16. doi: 10.1128/MCB.16.8.4107. Mol Cell Biol. 1996. PMID: 8754809 Free PMC article. - TEL/PDGFbetaR fusion protein activates STAT1 and STAT5: a common mechanism for transformation by tyrosine kinase fusion proteins.
Wilbanks AM, Mahajan S, Frank DA, Druker BJ, Gilliland DG, Carroll M. Wilbanks AM, et al. Exp Hematol. 2000 May;28(5):584-93. doi: 10.1016/s0301-472x(00)00138-7. Exp Hematol. 2000. PMID: 10812249 - TEL/platelet-derived growth factor receptor beta activates phosphatidylinositol 3 (PI3) kinase and requires PI3 kinase to regulate the cell cycle.
Dierov J, Xu Q, Dierova R, Carroll M. Dierov J, et al. Blood. 2002 Mar 1;99(5):1758-65. doi: 10.1182/blood.v99.5.1758. Blood. 2002. PMID: 11861293 - The TEL gene contributes to the pathogenesis of myeloid and lymphoid leukemias by diverse molecular genetic mechanisms.
Golub TR, Barker GF, Stegmaier K, Gilliland DG. Golub TR, et al. Curr Top Microbiol Immunol. 1997;220:67-79. doi: 10.1007/978-3-642-60479-9_5. Curr Top Microbiol Immunol. 1997. PMID: 9103676 Review. No abstract available. - Involvement of the TEL gene in hematologic malignancy by diverse molecular genetic mechanisms.
Golub TR, Barker GF, Stegmaier K, Gilliland DG. Golub TR, et al. Curr Top Microbiol Immunol. 1996;211:279-88. doi: 10.1007/978-3-642-85232-9_28. Curr Top Microbiol Immunol. 1996. PMID: 8585959 Review. No abstract available.
Cited by
- Polymerization of the SAM domain of TEL in leukemogenesis and transcriptional repression.
Kim CA, Phillips ML, Kim W, Gingery M, Tran HH, Robinson MA, Faham S, Bowie JU. Kim CA, et al. EMBO J. 2001 Aug 1;20(15):4173-82. doi: 10.1093/emboj/20.15.4173. EMBO J. 2001. PMID: 11483520 Free PMC article. - Potentiation of antileukemic therapies by the dual PI3K/PDK-1 inhibitor, BAG956: effects on BCR-ABL- and mutant FLT3-expressing cells.
Weisberg E, Banerji L, Wright RD, Barrett R, Ray A, Moreno D, Catley L, Jiang J, Hall-Meyers E, Sauveur-Michel M, Stone R, Galinsky I, Fox E, Kung AL, Griffin JD. Weisberg E, et al. Blood. 2008 Apr 1;111(7):3723-34. doi: 10.1182/blood-2007-09-114454. Epub 2008 Jan 9. Blood. 2008. PMID: 18184863 Free PMC article. - Gene expression-based screening for inhibitors of PDGFR signaling.
Antipova AA, Stockwell BR, Golub TR. Antipova AA, et al. Genome Biol. 2008;9(3):R47. doi: 10.1186/gb-2008-9-3-r47. Epub 2008 Mar 1. Genome Biol. 2008. PMID: 18312689 Free PMC article. - Activation of tyrosine kinases by mutation of the gatekeeper threonine.
Azam M, Seeliger MA, Gray NS, Kuriyan J, Daley GQ. Azam M, et al. Nat Struct Mol Biol. 2008 Oct;15(10):1109-18. doi: 10.1038/nsmb.1486. Epub 2008 Sep 14. Nat Struct Mol Biol. 2008. PMID: 18794843 Free PMC article. - Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion.
Lengline E, Beldjord K, Dombret H, Soulier J, Boissel N, Clappier E. Lengline E, et al. Haematologica. 2013 Nov;98(11):e146-8. doi: 10.3324/haematol.2013.095372. Haematologica. 2013. PMID: 24186319 Free PMC article. No abstract available.
References
- Claesson-Welsh L. J Biol Chem. 1994;269:32023–32026. - PubMed
- Shurtleff S, Buijs A, Behm F, Rubnitz J, Raimondi S, Hancock M, Chan G, Pui C, Grosveld G, Downing J. Leukemia. 1995;9:1985–1989. - PubMed
- Papadopoulos P, Ridge S A, Boucher C A, Stocking C, Wiedemann L M. Cancer Res. 1995;55:34–38. - PubMed
- Buijs A, Sherr S, van Baal S, van Bezouw S, van der Plas D, van Kessel A D, Riegman P, Deprez R L, Zwarthoff E, Hagemeijer A, Grosveld G. Oncogene. 1995;10:1511–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01DK50654-01/DK/NIDDK NIH HHS/United States
- P01 CA066996/CA/NCI NIH HHS/United States
- P01CA66996-01/CA/NCI NIH HHS/United States
- Wellcome Trust/United Kingdom
- P01 DK050654/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous