Aspergillus has distinct fatty acid synthases for primary and secondary metabolism - PubMed (original) (raw)
Aspergillus has distinct fatty acid synthases for primary and secondary metabolism
D W Brown et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Aspergillus nidulans contains two functionally distinct fatty acid synthases (FASs): one required for primary fatty acid metabolism (FAS) and the other required for secondary metabolism (sFAS). FAS mutants require long-chain fatty acids for growth, whereas sFAS mutants grow normally but cannot synthesize sterigmatocystin (ST), a carcinogenic secondary metabolite structurally and biosynthetically related to aflatoxin. sFAS mutants regain the ability to synthesize ST when provided with hexanoic acid, supporting the model that the ST polyketide synthase uses this short-chain fatty acid as a starter unit. The characterization of both the polyketide synthase and FAS may provide novel means for modifying secondary metabolites.
Figures
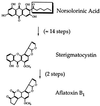
Figure 1
Sterigmatocystin/AF pathway. The aliphatic moiety of norsolorinic acid, the first intermediate in the pathway, is enclosed in a box.
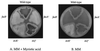
Figure 2
Growth requirements of the strains disrupted in the two FASs. From the top clockwise: TTAARG (wild type), TDB57.1 (_fasB_−), TDB5 (_stcK_−), TDB1 (_stcJ_−), and TDB51.1 (_fasA_−). Minimal media (B) and minimal media plus 2 mM myristic acid (_n_-tetradecanoic acid, Sigma) (A).
Figure 3
ST analysis of oatmeal culture extracts by TLC. Approximately 20 times as much material from_stcJ_− and _stcK_− mutants was loaded as compared with wild type and samples were resolved with benzene/glacial acetic acid (95:5 vol/vol). The nature of the band above ST is unknown.
Figure 4
Amino acid alignments of two representative FAS domains. (A) FAS β-keto acyl synthase domain of the α subunit. Ca, C. albicans (23); Sc, S. cerevisiae (25); Pp, P. patulum (28); and FasA and StcJ, A. nidulans. The constitutive FAS a peptides share 78% identity within this domain in contrast to StcJ that shares only 57% identity. (B) FAS malonyl/palmitoyl transferase domain of the β subunit. Ca, C. albicans (22); Sc,S. cerevisiae (24); Yl, Y. lipolytica (26); and FasB and StcK, A. nidulans. The constitutive FAS β peptides share 72% identity in contrast to StcK that shares 44% identity.
Similar articles
- Starter unit specificity directs genome mining of polyketide synthase pathways in fungi.
Crawford JM, Vagstad AL, Ehrlich KC, Townsend CA. Crawford JM, et al. Bioorg Chem. 2008 Feb;36(1):16-22. doi: 10.1016/j.bioorg.2007.11.002. Epub 2008 Jan 22. Bioorg Chem. 2008. PMID: 18215412 Free PMC article. - Hexanoate synthase, a specialized type I fatty acid synthase in aflatoxin B1 biosynthesis.
Hitchman TS, Schmidt EW, Trail F, Rarick MD, Linz JE, Townsend CA. Hitchman TS, et al. Bioorg Chem. 2001 Oct;29(5):293-307. doi: 10.1006/bioo.2001.1216. Bioorg Chem. 2001. PMID: 16256699 - Crosstalk between primary and secondary metabolism: Interconnected fatty acid and polyketide biosynthesis in prokaryotes.
West AR, Bailey CB. West AR, et al. Bioorg Med Chem Lett. 2023 Jul 15;91:129377. doi: 10.1016/j.bmcl.2023.129377. Epub 2023 Jun 14. Bioorg Med Chem Lett. 2023. PMID: 37328038 Free PMC article. Review. - Analysis of a mycotoxin gene cluster in Aspergillus nidulans.
Keller NP, Adams TH. Keller NP, et al. SAAS Bull Biochem Biotechnol. 1995;8:14-21. SAAS Bull Biochem Biotechnol. 1995. PMID: 7546572 Review. - Distribution of sterigmatocystin in filamentous fungi.
Rank C, Nielsen KF, Larsen TO, Varga J, Samson RA, Frisvad JC. Rank C, et al. Fungal Biol. 2011 Apr-May;115(4-5):406-20. doi: 10.1016/j.funbio.2011.02.013. Epub 2011 Feb 24. Fungal Biol. 2011. PMID: 21530923
Cited by
- Identification and molecular genetic analysis of the cichorine gene cluster in Aspergillus nidulans.
Sanchez JF, Entwistle R, Corcoran D, Oakley BR, Wang CC. Sanchez JF, et al. Medchemcomm. 2012 Aug 1;3(8):10.1039/C2MD20055D. doi: 10.1039/C2MD20055D. Medchemcomm. 2012. PMID: 24244835 Free PMC article. - Starter unit specificity directs genome mining of polyketide synthase pathways in fungi.
Crawford JM, Vagstad AL, Ehrlich KC, Townsend CA. Crawford JM, et al. Bioorg Chem. 2008 Feb;36(1):16-22. doi: 10.1016/j.bioorg.2007.11.002. Epub 2008 Jan 22. Bioorg Chem. 2008. PMID: 18215412 Free PMC article. - Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management.
Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. Kumar P, et al. Front Microbiol. 2017 Jan 17;7:2170. doi: 10.3389/fmicb.2016.02170. eCollection 2016. Front Microbiol. 2017. PMID: 28144235 Free PMC article. Review. - New Aspercryptins, Lipopeptide Natural Products, Revealed by HDAC Inhibition in Aspergillus nidulans.
Henke MT, Soukup AA, Goering AW, McClure RA, Thomson RJ, Keller NP, Kelleher NL. Henke MT, et al. ACS Chem Biol. 2016 Aug 19;11(8):2117-23. doi: 10.1021/acschembio.6b00398. Epub 2016 Jun 24. ACS Chem Biol. 2016. PMID: 27310134 Free PMC article. - Analysis of the Relationship between Alternative Respiration and Sterigmatocystin Formation in Aspergillus nidulans.
Molnár ÁP, Németh Z, Fekete E, Flipphi M, Keller NP, Karaffa L. Molnár ÁP, et al. Toxins (Basel). 2018 Apr 20;10(4):168. doi: 10.3390/toxins10040168. Toxins (Basel). 2018. PMID: 29677138 Free PMC article.
References
- Hutchinson C R, Fujii I. Annu Rev Microbiol. 1995;49:201–238. - PubMed
- Sargeant K, Sheridan A, O’Kelly J. Nature (London) 1961;192:1095–1097.
- Wogan, G. N., Househam, K. C. & Hundt, H. K. (1992) Cancer Res. 52, Suppl., 2114S–2118S. - PubMed
- Hsu I C, Metcalf R A, Sun T, Welsh J A, Wang N J, Harris C C. Nature (London) 1991;350:427–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous