ZnT-3, a putative transporter of zinc into synaptic vesicles - PubMed (original) (raw)
ZnT-3, a putative transporter of zinc into synaptic vesicles
R D Palmiter et al. Proc Natl Acad Sci U S A. 1996.
Abstract
The murine ZnT3 gene was cloned by virtue of its homology to the ZnT2 gene, which encodes a membrane protein that facilitates sequestration of zinc in endosomal vesicles. ZnT-3 protein is predicted to have six transmembrane domains and shares 52% amino acid identity with ZnT-2, with the homology extending throughout the two sequences. Human ZnT-3 cDNAs were also cloned; the amino acid sequence is 86% identical to murine ZnT-3. The mouse ZnT3 gene has 8 exons and maps to chromosome 5. Northern blot and reverse transcriptase-PCR analyses demonstrate that murine ZnT-3 expression is restricted to the brain and testis. In situ hybridization reveals that within the brain, ZnT-3 mRNA is most abundant in the hippocampus and cerebral cortex. Antibodies raised against the C-terminal tail of mouse ZnT-3 react with the projections from these neurons and produce a pattern similar to that obtained with Timm's reaction, which reveals histochemically reactive zinc within synaptic vesicles. We propose that ZnT-3 facilitates the accumulation of zinc in synaptic vesicles.
Figures
Figure 1
Comparison of mouse ZnT-3 protein sequence with rat ZnT-2. Amino acids are designated by one-letter code; identical amino acids are connected by lines; similar amino acids are connected by dots; the six predicted transmembrane domains are boxed with Roman numerals.
Figure 2
Sequence of mouse ZnT-3 cDNA, comparison of mouse and human ZnT-3 protein sequences, and mouse ZnT-3 genomic organization. (A) Mouse ZnT-3 cDNA sequence with amino acid sequence in three-letter code above. Differences between human and mouse ZnT-3 are indicated above the mouse protein sequence. Arrows indicate the location of introns and numbers designate the flanking exons. (B) A map of the mouse ZnT-3 genomic DNA showing the location of 8 exons. The regions that have been sequenced are indicated with a dotted line.
Figure 3
Organ distribution of ZnT-3, ZnT-2, and ZnT-1 mRNAs. Total RNA from the indicated organs was prepared and subjected to reverse transcription followed by PCR. The products were electrophoresed through 2.8% agarose and stained with ethidium bromide.
Figure 4
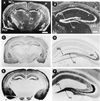
Localization of ZnT-3 mRNA, protein, and histochemically reactive zinc in the brain. Coronal sections of mouse brain were subjected to in situ hybridization with a33P-labeled probe complementary to mouse ZnT-3 mRNA (A and B), immunocytochemistry with affinity-purified antisera raised against the C-terminal tail of ZnT-3 (C and D), and Timm’s staining procedure for vesicular zinc (E and F). (B, D, and F, ×2.8; A, C, and_E_, ×0.7). In the hippocampus, ZnT-3 mRNA is present in the cell bodies of dentate granule cells, and ZnT-3 protein is abundant in the zinc-rich mossy fiber projections emanating from these neurons. A, amygdala (lateral nucleus); H, hippocampus; NCx, neocortex; PCx, piriform cortex; PV, paraventricular thalamic nucleus; ZI, zona inserta; GC, granule cell neurons of the dentate gyrus; hi, hilus; mf, mossy fibers. [Bars = 1000 μm (A) and 200 μm (B).]
Figure 5
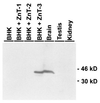
Western blot analysis of ZnT-3 protein. Lanes 1–4 represent equivalent amounts of protein isolated from confluent BHK cells: untransfected cells (lane 1), cells expressing ZnT-1 (lane 2), cells expressing ZnT-2 (lane 3), and cells expressing ZnT-3 (lane 4). Lanes 5–7 represent total protein from brain (lane 5), testis (lane 6), and kidney (lane 7). The amount of protein in all lanes was similar by Coomassie blue 250 staining of a replicate gel. Affinity-purified antibody to ZnT-3 was detected with a peroxidase-linked secondary antibody by chemiluminescence.
Similar articles
- Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey.
Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Wenzel HJ, et al. Proc Natl Acad Sci U S A. 1997 Nov 11;94(23):12676-81. doi: 10.1073/pnas.94.23.12676. Proc Natl Acad Sci U S A. 1997. PMID: 9356509 Free PMC article. - ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration.
Palmiter RD, Cole TB, Findley SD. Palmiter RD, et al. EMBO J. 1996 Apr 15;15(8):1784-91. EMBO J. 1996. PMID: 8617223 Free PMC article. - Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene.
Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Cole TB, et al. Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1716-21. doi: 10.1073/pnas.96.4.1716. Proc Natl Acad Sci U S A. 1999. PMID: 9990090 Free PMC article. - Mammalian zinc transporters.
McMahon RJ, Cousins RJ. McMahon RJ, et al. J Nutr. 1998 Apr;128(4):667-70. doi: 10.1093/jn/128.4.667. J Nutr. 1998. PMID: 9521625 Review. - Integrative aspects of zinc transporters.
Cousins RJ, McMahon RJ. Cousins RJ, et al. J Nutr. 2000 May;130(5S Suppl):1384S-7S. doi: 10.1093/jn/130.5.1384S. J Nutr. 2000. PMID: 10801948 Review.
Cited by
- Zinc and its binding proteins: essential roles and therapeutic potential.
Kiouri DP, Chasapis CT, Mavromoustakos T, Spiliopoulou CA, Stefanidou ME. Kiouri DP, et al. Arch Toxicol. 2024 Nov 7. doi: 10.1007/s00204-024-03891-3. Online ahead of print. Arch Toxicol. 2024. PMID: 39508885 Review. - Structural insights into human zinc transporter ZnT1 mediated Zn2+ efflux.
Long Y, Zhu Z, Zhou Z, Yang C, Chao Y, Wang Y, Zhou Q, Wang MW, Qu Q. Long Y, et al. EMBO Rep. 2024 Nov;25(11):5006-5025. doi: 10.1038/s44319-024-00287-3. Epub 2024 Oct 10. EMBO Rep. 2024. PMID: 39390258 Free PMC article. - Cell-type-specific enhancement of deviance detection by synaptic zinc in the mouse auditory cortex.
McCollum M, Manning A, Bender PTR, Mendelson BZ, Anderson CT. McCollum M, et al. Proc Natl Acad Sci U S A. 2024 Oct;121(40):e2405615121. doi: 10.1073/pnas.2405615121. Epub 2024 Sep 23. Proc Natl Acad Sci U S A. 2024. PMID: 39312661 - On the genesis and unique functions of zinc neuromodulation.
Bizup B, Tzounopoulos T. Bizup B, et al. J Neurophysiol. 2024 Oct 1;132(4):1241-1254. doi: 10.1152/jn.00285.2024. Epub 2024 Aug 28. J Neurophysiol. 2024. PMID: 39196675 Review. - In vitro reconstitution of transition metal transporters.
Ongey EL, Banerjee A. Ongey EL, et al. J Biol Chem. 2024 Aug;300(8):107589. doi: 10.1016/j.jbc.2024.107589. Epub 2024 Jul 19. J Biol Chem. 2024. PMID: 39032653 Free PMC article. Review.
References
- Vallee B L, Falchuk K H. Physiol Rev. 1993;73:79–117. - PubMed
- Palmiter, R. D. (1987) Experientia 52, Suppl., 63–80. - PubMed
- Zhao H, Eide D. J Biol Chem. 1996;271:23203–23210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases