Postischemic administration of adenosine amine congener (ADAC): analysis of recovery in gerbils - PubMed (original) (raw)
Postischemic administration of adenosine amine congener (ADAC): analysis of recovery in gerbils
D K Von Lubitz et al. Eur J Pharmacol. 1996.
Abstract
Although adenosine receptor-based treatment of cerebral ischemia and other neurodegenerative disorders has been frequently advocated, cardiovascular side effects and an uncertain therapeutic time window of such treatment have constituted major obstacles to clinical implementation. Therefore, we have investigated the neuroprotective effects of the adenosine A1 receptor agonist adenosine amine congener (ADAC) injected after either 5 or 10 min ischemia at 100 micrograms/kg. When the drug was administered at either 6 or 12 h following 5 min forebrain ischemia, all animals were still alive on the 14th day after the occlusion. In both ADAC treated groups neuronal survival was approximately 85% vs. 50% in controls. Administration of a single dose of ADAC at times 15 min to 12 h after 10 min ischemia resulted in a significant improvement of survival in animals injected either at 15 or 30 min, or at 1, 2, or 3 h after the insult. In all 10 min ischemia groups, administration of ADAC resulted in a significant protection of neuronal morphology and preservation of microtubule associated protein 2 (MAP-2). However, postischemic Morris' water maze tests revealed full preservation of spatial memory and learning ability in animals injected at 6 h. On the other hand, the performance of gerbils treated at 12 h postischemia was indistinguishable from that of the controls. Administration of ADAC at 100 micrograms/kg in non-ischemic animals did not result in bradycardia, hypotension, or hypothermia. The data indicate that when ADAC is used postischemically, the most optimal level of protection is obtained when drugs are given at 30 min to 6 h after the insult. Although the mechanisms involved in neuroprotective effects of adenosine A1 receptor agonists require further studies, the present results demonstrate the feasibility of their clinical applications.
Figures
Fig. 1
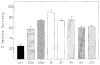
Survival of 10 min ischemia. Symbols: black squares – controls; open squares – ADAC 15 min post; open triangles – ADAC 30 min post; open circles – ADAC 1 h post; open diamonds – ADAC 2 h post; black circles – ADAC 3 h post; black triangles – ADAC 6 h post; asterisks –ADAC 12 h post. Only groups given ADAC at 15, 30, 60 and 120 min are statistically different from the controls (P < 0.05. Bonferroni corrected Fisher’s test).
Fig. 2
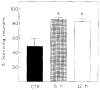
Neuronal survival following 5 min ischemia. a P < 0.05. Bars: S.E.M.
Fig. 3
Neuronal survival following 10 min ischemia. Groups injected at 30 min or at 1, 2 or 3 h postischemia are statistically different from all other treatment groups (P < 0.05, Student-Newman-Keuls test). Bars: S.E.M.
Fig. 4
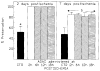
MAP 2 preservation at 2 or 7 days postischemia. a P < 0.05 vs. all treatment groups; b P < 0.05 treatment groups at 2 days vs. 7 days postischemia (Student-Newman-Keuls test). Bars: S.E.M.
Fig. 5
Immunofluorescent staining pattern of MAP 2 (monoclonal antibody linked to Texas Red) in the hippocampal CA1 sector of a normal gerbil (A). Seven days after 10 min ischemia, the destruction of MAP 2 is evident (B). Seven days following postischemic treatment with ADAC (100 μg/kg, 2 h after 10 min ischemia) results in extensive preservation of this cytoskeletal protein (C). Arrows: dendrites with normal appearance of MAP-2.
Fig. 6
Target latencies prior to and 14 days after ischemia. Single factor repeated measures ANOVA revealed significant main effects of treatment group (F(2,21)= 13.261, P< 0.001) and trial days (F(9,189) = 5.599, P < 0.001). No significant interaction between treatment group and trial days was observed (F(1, 189) = 0.805, P = 0.693). Symbols: asterisk –the last preischemic trial; black squares – controls; open circles – ADAC at 6 h postischemia; open squares – ADAC at 12 h postischemia.
Fig. 7
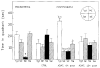
Probe trials (quadrant preference) pre- and after ischemia. Single factor repeated measures ANOVA revealed no significant main effect of treatment group (F(2,21) = 0.189, P = 0.83) or quadrant latency (F(3,63) = 2.376, P < 0.078). However, a significant interaction between treatment group and quadrant latency was observed (F(6,63) = 4.272, P < 0.001). Abbreviations: a P < 0.05 within the group; b P < 0.05 between pre- and postischemic groups; c P < 0.05 between postischemic controls and treatment groups. Quadrant designations: Tgt – target; NE –north/east; SE – south/east; SW – south/west quadrants. Inset: plan of the maze.
Similar articles
- Protection against ischemic damage by adenosine amine congener, a potent and selective adenosine A1 receptor agonist.
Von Lubitz DK, Lin RC, Bischofberger N, Beenhakker M, Boyd M, Lipartowska R, Jacobson KA. Von Lubitz DK, et al. Eur J Pharmacol. 1999 Mar 26;369(3):313-7. doi: 10.1016/s0014-2999(99)00073-4. Eur J Pharmacol. 1999. PMID: 10225368 Free PMC article. - Reduction of postischemic brain damage and memory deficits following treatment with the selective adenosine A1 receptor agonist.
Von Lubitz DK, Beenhakker M, Lin RC, Carter MF, Paul IA, Bischofberger N, Jacobson KA. Von Lubitz DK, et al. Eur J Pharmacol. 1996 Apr 29;302(1-3):43-8. doi: 10.1016/0014-2999(96)00101-x. Eur J Pharmacol. 1996. PMID: 8790990 Free PMC article. - Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist.
Von Lubitz DK, Lin RC, Jacobson KA. Von Lubitz DK, et al. Eur J Pharmacol. 1995 Dec 20;287(3):295-302. doi: 10.1016/0014-2999(95)00498-x. Eur J Pharmacol. 1995. PMID: 8991804 Free PMC article. - Adenosine as a Key Mediator of Neuronal Survival in Cerebral Ischemic Injury.
Khan H, Kaur P, Singh TG, Grewal AK, Sood S. Khan H, et al. Neurochem Res. 2022 Dec;47(12):3543-3555. doi: 10.1007/s11064-022-03737-3. Epub 2022 Aug 30. Neurochem Res. 2022. PMID: 36042141 Review. - Stimulation of adenosine A3 receptors in cerebral ischemia. Neuronal death, recovery, or both?
von Lubitz DK, Ye W, McClellan J, Lin RC. von Lubitz DK, et al. Ann N Y Acad Sci. 1999;890:93-106. doi: 10.1111/j.1749-6632.1999.tb07984.x. Ann N Y Acad Sci. 1999. PMID: 10668416 Review.
Cited by
- A neoceptor approach to unraveling microscopic interactions between the human A2A adenosine receptor and its agonists.
Jacobson KA, Ohno M, Duong HT, Kim SK, Tchilibon S, Cesnek M, Holý A, Gao ZG. Jacobson KA, et al. Chem Biol. 2005 Feb;12(2):237-47. doi: 10.1016/j.chembiol.2004.12.010. Chem Biol. 2005. PMID: 15734651 Free PMC article. - The adenosine A1 receptor agonist adenosine amine congener exerts a neuroprotective effect against the development of striatal lesions and motor impairments in the 3-nitropropionic acid model of neurotoxicity.
Blum D, Gall D, Galas MC, d'Alcantara P, Bantubungi K, Schiffmann SN. Blum D, et al. J Neurosci. 2002 Oct 15;22(20):9122-33. doi: 10.1523/JNEUROSCI.22-20-09122.2002. J Neurosci. 2002. PMID: 12388620 Free PMC article. - Protection against ischemic damage by adenosine amine congener, a potent and selective adenosine A1 receptor agonist.
Von Lubitz DK, Lin RC, Bischofberger N, Beenhakker M, Boyd M, Lipartowska R, Jacobson KA. Von Lubitz DK, et al. Eur J Pharmacol. 1999 Mar 26;369(3):313-7. doi: 10.1016/s0014-2999(99)00073-4. Eur J Pharmacol. 1999. PMID: 10225368 Free PMC article. - Adenosine receptors as therapeutic targets.
Jacobson KA, Gao ZG. Jacobson KA, et al. Nat Rev Drug Discov. 2006 Mar;5(3):247-64. doi: 10.1038/nrd1983. Nat Rev Drug Discov. 2006. PMID: 16518376 Free PMC article. Review. - Adenosine A1 receptor agonists as clinically viable agents for treatment of ischemic brain disorders.
Bischofberger N, Jacobson KA, von Lubitz DK. Bischofberger N, et al. Ann N Y Acad Sci. 1997 Oct 15;825:23-9. doi: 10.1111/j.1749-6632.1997.tb48411.x. Ann N Y Acad Sci. 1997. PMID: 9369972 Free PMC article. Review. No abstract available.
References
- Andiné P. Involvement of adenosine in ischemic and postischemic calcium regulation. Mol Chem Neuropathol. 1993;18:35. - PubMed
- Boyd M, Meshulam Y, Jacobson KA, Von Lubitz DKJE. FASEB J. Washington, DC: 1996. Apr 14–17, Effect of A1 adenosine receptor agonist and antagonist on [3H]glutamic acid release from cortical tissue in vitro. Abstracts 10, A159, Abstract 920.
- Busto R, Dietrich WD, Globus MYT, Ginsberg MD. The importance of brain temperature in cerebral ischemic injury. Stroke. 1989;20:1113. - PubMed
- Deckert J, Gleiter CH. Adenosine – an endogenous neuroprotective metabolite and neuromodulator. J Neural Transm (Suppl) 1994;43:23. - PubMed
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988;9:130. - PubMed
MeSH terms
Substances
Grants and funding
- Z99 DA999999/ImNIH/Intramural NIH HHS/United States
- Z01 DK031117/ImNIH/Intramural NIH HHS/United States
- Z01 DK031117-20/ImNIH/Intramural NIH HHS/United States
- Z99 OD999999/ImNIH/Intramural NIH HHS/United States
- Z99 DK999999/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources