Inhibiting transthyretin amyloid fibril formation via protein stabilization - PubMed (original) (raw)
Inhibiting transthyretin amyloid fibril formation via protein stabilization
G J Miroy et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Transthyretin (TTR) amyloid fibril formation is observed systemically in familial amyloid polyneuropathy and senile systemic amyloidosis and appears to be the causative agent in these diseases. Herein, we demonstrate conclusively that thyroxine (10.8 microM) inhibits TTR fibril formation efficiently in vitro and does so by stabilizing the tetramer against dissociation and the subsequent conformational changes required for amyloid fibril formation. In addition, the nonnative ligand 2,4,6-triiodophenol, which binds to TTR with slightly increased affinity also inhibits TTR fibril formation by this mechanism. Sedimentation velocity experiments were employed to show that TTR undergoes dissociation (linked to a conformational change) to form the monomeric amyloidogenic intermediate, which self-assembles into amyloid in the absence, but not in the presence of thyroxine. These results demonstrate the feasibility of using small molecules to stabilize the native fold of a potentially amyloidogenic human protein, thus preventing the conformational changes, which appear to be the common link in several human amyloid diseases. This strategy and the compounds resulting from further development should prove useful for critically evaluating the amyloid hypothesis--i.e., the putative cause-and-effect relationship between TTR amyloid deposition and the onset of familial amyloid polyneuropathy and senile systemic amyloidosis.
Figures
Figure 1
Schematic representation of the acid-mediated denaturation/amyloid fibril-forming pathway of TTR.
Figure 2
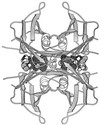
Ribbon diagram of tetrameric TTR illustrating the hourglass-shaped channel that runs through the center of the protein and serves as the binding site for T4 (3,3′-diiodothyroxine shown) molecules rendered as a space-filling structure (21). The four conserved water molecules are also shown in CPK (Corey–Pauling Space Filling Models) format.
Figure 3
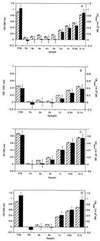
Inhibition of TTR amyloid fibril formation by T4 and TIP. The extent of fibril formation in the presence and absence of inhibitor at pH 4.4 was probed at 72 h by an optical density measurement at 330 nm (hatched bars) and by a quantitative Congo red binding assay (solid bars). (A) Wild-type fibril formation. From left to right, fibril formation in the absence of T4 and in the presence of T4 alone (no TTR) and TTR fibril formation in the presence of the indicated equivalents of T4 (3 eq of T4 = 10.8 μM) are shown. Inhibition of (B) V-30-M and (C) L-55-P TTR by T4 at pH 5.0. (D) Inhibition of wild-type TTR by TIP. The labeling of the axes and the organization of the data in_B_–D is the same as that in_A_.
Figure 4
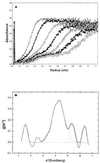
(A) Sedimentation velocity profile of wild-type TTR in the absence of T4 at pH 4.4. Solute distribution recorded at 235 nm (60,000 rpm). Scans for analysis were recorded every 3 min; for simplicity, only scans from every 24 min are shown. (B) Apparent sedimentation coefficient distribution (g*(s)t) as a function of sedimentation coefficient for wild-type TTR (0.2 mg/ml) in the absence of T4, indicating the formation of multiple species during the partial acid denaturation of TTR.
Figure 5
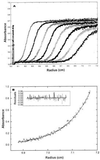
(A) Sedimentation velocity profile of wild-type TTR in the presence of T4 at pH 4.4. Solute distribution recorded at 235 nm (60,000 rpm). Scans for analysis were recorded every 3 min; for simplicity, only scans from every 15 min are shown. (B) Sedimentation equilibrium analysis of wild-type TTR at pH 4.4 in the presence of 10.8 μM. The solid line drawn through the data was obtained by fitting the absorbance vs. radial position_r_ to Eq. 2 for a homogeneous tetrameric species. The residual difference between the experimental data and the fitted data for each point is shown in the Inset.
Similar articles
- Inhibiting transthyretin conformational changes that lead to amyloid fibril formation.
Peterson SA, Klabunde T, Lashuel HA, Purkey H, Sacchettini JC, Kelly JW. Peterson SA, et al. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):12956-60. doi: 10.1073/pnas.95.22.12956. Proc Natl Acad Sci U S A. 1998. PMID: 9789022 Free PMC article. - Chromium(III) ion and thyroxine cooperate to stabilize the transthyretin tetramer and suppress in vitro amyloid fibril formation.
Sato T, Ando Y, Susuki S, Mikami F, Ikemizu S, Nakamura M, Suhr O, Anraku M, Kai T, Suico MA, Shuto T, Mizuguchi M, Yamagata Y, Kai H. Sato T, et al. FEBS Lett. 2006 Jan 23;580(2):491-6. doi: 10.1016/j.febslet.2005.12.047. Epub 2005 Dec 22. FEBS Lett. 2006. PMID: 16386248 - Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid.
Kelly JW, Colon W, Lai Z, Lashuel HA, McCulloch J, McCutchen SL, Miroy GJ, Peterson SA. Kelly JW, et al. Adv Protein Chem. 1997;50:161-81. doi: 10.1016/s0065-3233(08)60321-6. Adv Protein Chem. 1997. PMID: 9338081 Review. - Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses.
Johnson SM, Wiseman RL, Sekijima Y, Green NS, Adamski-Werner SL, Kelly JW. Johnson SM, et al. Acc Chem Res. 2005 Dec;38(12):911-21. doi: 10.1021/ar020073i. Acc Chem Res. 2005. PMID: 16359163 Review.
Cited by
- Impact of quaternary structure dynamics on allosteric drug discovery.
Jaffe EK. Jaffe EK. Curr Top Med Chem. 2013;13(1):55-63. doi: 10.2174/1568026611313010006. Curr Top Med Chem. 2013. PMID: 23409765 Free PMC article. - The flavonoid luteolin, but not luteolin-7-O-glucoside, prevents a transthyretin mediated toxic response.
Iakovleva I, Begum A, Pokrzywa M, Walfridsson M, Sauer-Eriksson AE, Olofsson A. Iakovleva I, et al. PLoS One. 2015 May 28;10(5):e0128222. doi: 10.1371/journal.pone.0128222. eCollection 2015. PLoS One. 2015. PMID: 26020516 Free PMC article. - Transthyretin Misfolding, A Fatal Structural Pathogenesis Mechanism.
Si JB, Kim B, Kim JH. Si JB, et al. Int J Mol Sci. 2021 Apr 23;22(9):4429. doi: 10.3390/ijms22094429. Int J Mol Sci. 2021. PMID: 33922648 Free PMC article. Review. - Small-molecule-mediated stabilization of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants against unfolding and aggregation.
Ray SS, Nowak RJ, Brown RH Jr, Lansbury PT Jr. Ray SS, et al. Proc Natl Acad Sci U S A. 2005 Mar 8;102(10):3639-44. doi: 10.1073/pnas.0408277102. Epub 2005 Feb 28. Proc Natl Acad Sci U S A. 2005. PMID: 15738401 Free PMC article. - A novel therapeutic strategy for polyglutamine diseases by stabilizing aggregation-prone proteins with small molecules.
Tanaka M, Machida Y, Nukina N. Tanaka M, et al. J Mol Med (Berl). 2005 May;83(5):343-52. doi: 10.1007/s00109-004-0632-2. Epub 2005 Mar 10. J Mol Med (Berl). 2005. PMID: 15759103 Review.
References
- Blake C C F, Geisow M J, Oatley S J. J Mol Biol. 1978;121:339–356. - PubMed
- Kisilevsky R. Lab Invest. 1983;49:381–390. - PubMed
- Castano E M, Frangione B. Lab Invest. 1988;58:122–132. - PubMed
- Benson M D. Trends Biochem Sci. 1989;12:88–92. - PubMed
- Jacobson D R, Buxbaum J N. Adv Hum Genet. 1991;20:69–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous