A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin - PubMed (original) (raw)
A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin
P Cesare et al. Proc Natl Acad Sci U S A. 1996.
Abstract
Pain differs from other sensations in many respects. Primary pain-sensitive neurons respond to a wide variety of noxious stimuli, in contrast to the relatively specific responses characteristic of other sensory systems, and the response is often observed to sensitize on repeated presentation of a painful stimulus, while adaptation is typically observed in other sensory systems. In most cases the cellular mechanisms of transduction and sensitization in response to painful stimuli are not understood. We report here that application of pulses of noxious heat to a subpopulation of isolated primary sensory neurons rapidly activates an inward current. The ion channel activated by heat discriminates poorly among alkali cations. Calcium ions both carry current and partially suppress the current carried by other ions. The current is markedly increased by bradykinin, a potent algogenic nonapeptide that is known to be released in vivo by tissue damage. Phosphatase inhibitors prolong the sensitization caused by bradykinin, and a similar sensitization is caused by activators of protein kinase C. We conclude that bradykinin sensitizes the response to heat by activating protein kinase C.
Figures
Figure 1
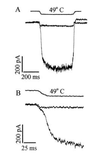
Activation of an inward current by heat in primary sensory neurons. (A) Membrane current activated in a heat-sensitive and a heat-insensitive neuron in response to a temperature change from 25°C to 49°C (timing of solution change given by top trace, obtained by measuring time course of change in solution conductance at pipette tip after detaching from the cell at the end of the experiment). In 56% (n = 155) of small neurons (<25 μm in diameter), a delayed increase in membrane current exceeding 50 pA was observed in response to a 49°C pulse; such neurons were classified as heat-sensitive. (B) Same traces as in A expanded to show delayed onset of current increase in heat-sensitive neurons.
Figure 2
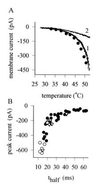
(A) Peak current as a function of temperature activated in a heat-sensitive neuron by 400-msec step temperature changes (•). A similar relation was obtained by the quicker procedure of applying a temperature ramp (trace 1). Trace 2 shows the result of a similar temperature ramp applied to a heat-insensitive neuron. (B) Relation between current activated by a 49°C temperature step and time to half-activation of delayed current in individual neurons. •, Untreated heat-sensitive neurons; ▵, neurons exposed to BK (1 μM for 20 sec; for further details, see Fig. 3). ○, Neurons exposed to the PKC activator PMA (1 μM for 20 sec; for further details, see Fig. 4).
Figure 4
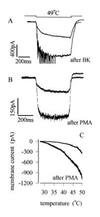
(A) Sensitization of heat response by BK. Traces show membrane current responses to steps to 49°C before and after application of BK (1 μM for 20 sec, which generated a transient inward current of maximum amplitude 203 pA in this neuron). Brief transient inward currents observed on application of heat after BK are action potentials elicited in unclamped neuronal processes. (B) Sensitization caused by application of the PKC activator PMA (1 μM for 20 sec). Mean increase in heat-sensitive current elicited by a 49°C temperature step was 532 ± 123% of control (n = 9), and significant increases were observed in all heat-sensitive neurons tested. PMA had no detectable effect on membrane current at room temperature in the majority of neurons. Experimental details as in A. (C) Current versus temperature relations, obtained using temperature ramps as in Fig. 2_A_, before and after application of PMA (1 μM).
Figure 3
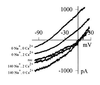
Current–voltage relations of heat-sensitive current, obtained by applying a voltage ramp passing from +30 mV to −100 mV in 455 msec during a brief (600 msec) temperature step to 49°C, and subtracting current observed in response to the ramp at room temperature. Heat-sensitive current was maximized for the purpose of these experiments by prior activation of PKC using PMA (1 μM) and inhibition of phosphatases using calyculin A (20 nM; see Figs. 4 and 5). Relations shown were obtained in the following order: in 140 mM [Na]o, 2 mM [Ca]o (mean_E_rev = −2 ± 1 mV,n = 9 cells); in 140 mM [Na]o, 0 mM [Ca]o (_E_rev = −4 ± 3 mV); in 0 mM [Na]o, 0 mM [Ca]o, using Na+ substitute_N_-methyl-
d
-glucamine (_E_rev = −69 ± 2 mV); in 0 mM [Na]o, 2 mM [Ca]o (_E_rev = −36 ± 1 mV); and again in 140 mM [Na]o, 2 mM [Ca]o (trace labeled “rec”).
Figure 5
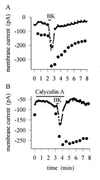
(A) Time course of sensitization induced by BK (1 μM). Simultaneous recording of inward current induced by BK (points) and current increase elicited by a 400 msec step to 49°C, repeated every 30 sec (•). Responses to temperature steps removed from current trace. Similar results obtained in 12 neurons. (B) Similar experiment in which the phosphatase inhibitor calyculin A (20 nM), which inhibits type 1 and 2A serine/threonine phosphatases, was applied before the BK pulse. Inward current induced by BK and sensitization of the heat response were unaffected, but recovery from the sensitized state was prevented by phosphatase inhibition. Similar results obtained in six neurons. The heat response was tested again 12 min after BK application and remained elevated at a level of 185 pA.
Figure 6
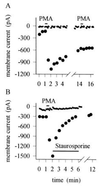
(A) Time course of sensitization induced by PMA. Time of application of PMA (1 μM) shown by bar. Similar results obtained in nine neurons. Interruptions in membrane current trace occur where test pulses of heat were applied. (B) Reversal of sensitization by the PKC inhibitor staurosporine. Similar experiment to that in A, but subsequent application of staurosporine (0.25 μM) as shown abolished the sensitization. Similar results obtained in five neurons. In separate experiments, application of staurosporine 2 min before PMA abolished the sensitization caused by PMA (n = 10).
Comment in
- More sensory competence for nociceptive neurons in culture.
Kress M, Reeh PW. Kress M, et al. Proc Natl Acad Sci U S A. 1996 Dec 24;93(26):14995-7. doi: 10.1073/pnas.93.26.14995. Proc Natl Acad Sci U S A. 1996. PMID: 8986750 Free PMC article. Review. No abstract available.
Similar articles
- Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat.
Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Cesare P, et al. Neuron. 1999 Jul;23(3):617-24. doi: 10.1016/s0896-6273(00)80813-2. Neuron. 1999. PMID: 10433272 - Inward currents in primary nociceptive neurons of the rat and pain sensations in humans elicited by infrared diode laser pulses.
Greffrath W, Nemenov MI, Schwarz S, Baumgärtner U, Vogel H, Arendt-Nielsen L, Treede RD. Greffrath W, et al. Pain. 2002 Sep;99(1-2):145-55. doi: 10.1016/s0304-3959(02)00071-4. Pain. 2002. PMID: 12237192 - More sensory competence for nociceptive neurons in culture.
Kress M, Reeh PW. Kress M, et al. Proc Natl Acad Sci U S A. 1996 Dec 24;93(26):14995-7. doi: 10.1073/pnas.93.26.14995. Proc Natl Acad Sci U S A. 1996. PMID: 8986750 Free PMC article. Review. No abstract available. - Excitation and sensitization of nociceptors by bradykinin: what do we know?
Mizumura K, Sugiura T, Katanosaka K, Banik RK, Kozaki Y. Mizumura K, et al. Exp Brain Res. 2009 Jun;196(1):53-65. doi: 10.1007/s00221-009-1814-5. Epub 2009 Apr 26. Exp Brain Res. 2009. PMID: 19396590 Review.
Cited by
- A journey from molecule to physiology and in silico tools for drug discovery targeting the transient receptor potential vanilloid type 1 (TRPV1) channel.
Amaya-Rodriguez CA, Carvajal-Zamorano K, Bustos D, Alegría-Arcos M, Castillo K. Amaya-Rodriguez CA, et al. Front Pharmacol. 2024 Jan 24;14:1251061. doi: 10.3389/fphar.2023.1251061. eCollection 2023. Front Pharmacol. 2024. PMID: 38328578 Free PMC article. Review. - A phenotypic screening platform for chronic pain therapeutics using all-optical electrophysiology.
Liu PW, Zhang H, Werley CA, Pichler M, Ryan SJ, Lewarch CL, Jacques J, Grooms J, Ferrante J, Li G, Zhang D, Bremmer N, Barnett A, Chantre R, Elder AE, Cohen AE, Williams LA, Dempsey GT, McManus OB. Liu PW, et al. Pain. 2024 Apr 1;165(4):922-940. doi: 10.1097/j.pain.0000000000003090. Epub 2023 Nov 14. Pain. 2024. PMID: 37963235 - Negative Modulation of TRPM8 Channel Function by Protein Kinase C in Trigeminal Cold Thermoreceptor Neurons.
Rivera B, Campos M, Orio P, Madrid R, Pertusa M. Rivera B, et al. Int J Mol Sci. 2020 Jun 22;21(12):4420. doi: 10.3390/ijms21124420. Int J Mol Sci. 2020. PMID: 32580281 Free PMC article. - Mapping the binding site of TRPV1 on AKAP79: implications for inflammatory hyperalgesia.
Btesh J, Fischer MJM, Stott K, McNaughton PA. Btesh J, et al. J Neurosci. 2013 May 22;33(21):9184-9193. doi: 10.1523/JNEUROSCI.4991-12.2013. J Neurosci. 2013. PMID: 23699529 Free PMC article. - Supercooling agent icilin blocks a warmth-sensing ion channel TRPV3.
Sherkheli MA, Gisselmann G, Hatt H. Sherkheli MA, et al. ScientificWorldJournal. 2012;2012:982725. doi: 10.1100/2012/982725. Epub 2012 Apr 1. ScientificWorldJournal. 2012. PMID: 22548000 Free PMC article.
References
- Besson J M, Chaouch A. Physiol Rev. 1987;67:67–155. - PubMed
- Treede R D, Meyer R A, Raja S N, Campbell J N. Prog Neurobiol. 1992;38:397–421. - PubMed
- Meyer R A, Campbell J N, Srinivasa N R. In: Textbook of Pain. 3rd Ed. Melzack R, Wall P, editors. Edinburgh: Churchill Livingstone; 1994. pp. 13–44.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical