Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody - PubMed (original) (raw)
Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody
G D'Arcangelo et al. J Neurosci. 1997.
Abstract
The neurological mouse mutant strain reeler displays abnormal laminar organization of several brain structures as a consequence of a defect in cell migration during neurodevelopment. This phenotype is a result of the disruption of reelin, a gene encoding a protein that has several structural characteristics of extracellular matrix proteins. To understand the molecular basis of the action of Reelin on neuronal migration, we constructed a full-length reelin clone and used it to direct Reelin expression. Here, we demonstrate that Reelin is a secreted glycoprotein and that a highly charged C-terminal region is essential for secretion. In addition, we demonstrate that an amino acid sequence present in the N-terminal region of Reelin contains an epitope that is recognized by the CR-50 monoclonal antibody. CR-50 was raised against an antigen expressed in normal mouse brain that is absent in reeler mice. The interaction of CR-50 with its epitope leads to the disruption of neural cell aggregation in vitro. Here, we used CR-50 to precipitate Reelin from reticulocyte extracts programmed with reelin mRNA, from cells transfected with reelin clones, and from cerebellar explants. The reelin gene product seems to function as an instructive signal in the regulation of neuronal migration.
Figures
Fig. 1.
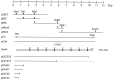
Assembly of full-length and truncated_reelin_ cDNAs. Five overlapping plasmids (lines) encoding portions of the _reelin_cDNA (p5BS1, pBS2, pBS6, p3Rea3, and pBS53) were digested with the indicated restriction enzymes and cloned consecutively into pcDNA3 to generate a full-length clone that contains the entire open reading frame under the control of the mammalian CMV and the bacteriophage T7 promoters (pCrl). The initiator methionine codon (Met) and the stop codon (Stop) are indicated. After signal peptide cleavage, the full-length protein (box) is predicted to be 385 kDa in size. EGF-like repeats (E, black boxes) and a highly positively charged region in the C terminus (++) are indicated. A double-stranded oligonucleotide encoding a c-Myc epitope, 9E10 (c-Myc), was cloned in frame into the unique_Bsp_EI restriction site of pCrl to generate the pCrlM construct. Other expression constructs (lines) encoding truncated Reelin proteins in pcDNA3 are shown below the protein diagram. The numbers contained in the expression construct names refer to the last encoded amino acid of Reelin. The pCrl1837 contains an inverted c-Myc oligonucleotide at the_Bsp_EI restriction site, which puts the C-terminal half of the protein out of frame (dotted line).
Fig. 2.
Expression of full-length Reelin in vitro. A plasmid encoding full-length Myc-tagged Reelin (pCrlM) was transcribed in vitro by the T7 RNA polymerase and translated by using a rabbit reticulocyte lysate. The [35S]-labeled final product was analyzed on a 4–12% SDS-polyacrylamide gel. Aliquots of the reaction were treated with no antibody (lane 1), monoclonal antibody against c-Myc (9E10, lane 2), monoclonal antibody CR-50 (lane 3), polyclonal anti-Reelin peptide Rlp3 (lane 4), or preimmune serum (lane 5). The immunoprecipitated Reelin protein is ∼385 kDa.
Fig. 3.
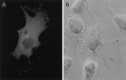
Intracellular localization of Reelin in COS cells. After transfection with pCrlM encoding full-length Reelin under the control of the CMV promoter, COS cells were fixed, permeabilized, and incubated for 1 hr with the primary CR-50 antibody and for an additional 30 min with FITC-tagged anti-mouse secondary antibody.A, Fluorescent image of a cell expressing Reelin (80× magnification). B, Phase-contrast image of the same cell field.
Fig. 4.
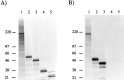
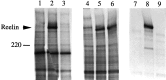
CR-50 epitope mapping. Plasmids encoding truncated Reelin proteins were transcribed in vitro by the T7 RNA polymerase and translated by using a rabbit reticulocyte lysate in the presence of [35S]methionine and [35S]cysteine. A, The products of pCrl1837 (206 kDa, lane 1), pCrl462 (51 kDa, lane 2), pCrl407 (46 kDa, lane 3), pCrl250 (27.5 kDa,lane 4), and pCrl194 (22 kDa, lane 5) were analyzed on a 4–12% SDS-polyacrylamide gel.B, The same in vitro translated proteins as in A were subjected to immunoprecipitation with the monoclonal antibody CR-50 and analyzed on a 4–12% SDS-polyacrylamide gel. Because all truncated proteins except those encoded by pCrl194 and pCrl250 are recognized by CR-50, the epitope is located between amino acids 251 and 407 of Reelin.
Fig. 5.
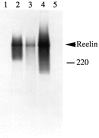
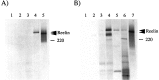
Reelin is modified and secreted by COS or cerebellar cells. A, COS cells (lanes 1–4) were transfected with (lanes 3, 4) or without (lanes 1, 2) pCrlM. The [35S]-labeled supernatant was subjected to immunoprecipitation with no antibody (lanes 1, 3) or with anti-Myc antibody 9E10 (lanes 2, 4). A specific band of ∼400 kDa (top arrowhead) was detected only in the sample containing both pCrlM and anti-Myc. The predicted 385 kDa in vitro translated [35S]-labeled product of pCrlM was immunoprecipitated with the anti-Myc antibody (lane 5). The protein produced in vitro (bottom arrowhead) seems to have a lower molecular mass than that obtained in COS cells. B, The supernatant from COS cells transfected with no DNA (lanes 1, 2) or pCrlM (lanes 3, 4), cerebellar cell supernatant (lane 5) or cerebellar cell lysates (lane 6), and in vitro translation reaction of pCrlM (lane 7) were labeled with [35S], as described in Materials and Methods, and subjected to immunoprecipitation with the CR-50 antibody. All immunoprecipitates from transfected COS or cerebellar cells migrated at a higher apparent molecular weight (top arrowhead) than the in vitro translated product (bottom arrowhead). The position of a 220 kDa molecular weight marker is indicated.
Fig. 6.
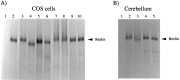
Reelin is glycosylated. A, COS cells were transfected with no DNA (lane 1) or with pCrlM (lanes 2–10). Cells were labeled with [35S], and Reelin was immunoprecipitated from the supernatant with the CR-50 antibody. Immunocomplexes were resuspended in gel loading buffer (lanes 1, 2) or in the appropriate glycosidase buffer and incubated with (lane 4) or without (lane 3) PNGaseF, with (lane 6) or without (lane 5)_O_-glycosidase, with (lane 8) or without (lane 7) neuraminidase A, and with (lane 10) or without (lane 9) chondroitinase ABC. After digestion, gel loading buffer was added, and the samples were analyzed on a 4% SDS polyacrylamide gel. B, Cerebellar cells were obtained from postnatal day 7 reeler(lane 1) or normal (lanes 2–5) mice. Cells were labeled with [35S], and Reelin was immunoprecipitated from the supernatant with the CR-50 antibody. Immunocomplexes were resuspended in gel loading buffer (lanes 1, 2) or in the appropriate glycosidase buffer and digested with PNGaseF (lane 3), neuraminidase A (lane 4), or _O_-glycosidase (lane 5). Samples were analyzed on a 4% SDS polyacrylamide gel.
Fig. 7.
The C terminus of Reelin is required for secretion. COS cells were transfected with no DNA (lanes 1, 4, 7), pCrlM (lanes 2, 5, 8), or pCrl3328 (lanes 3, 6, 9) and labeled with [35S]. Lysates (lanes 1–6) or supernatants (lanes 7–9) were subjected to immunoprecipitation with anti-rp5 (lanes 1–3) or with CR-50 antibody (lanes 4–9). The anti-rp5 antibody, directed against the C terminus of Reelin, recognized the full-length product of pCrl, but not the truncated product of pCrl3328. CR-50 immunoprecipitated both proteins from the cell lysate, but it only precipitated the full-length protein from the cell supernatant. The migration of a 220 kDa molecular weight marker is indicated.
Similar articles
- Role of reelin in the control of brain development.
Curran T, D'Arcangelo G. Curran T, et al. Brain Res Brain Res Rev. 1998 May;26(2-3):285-94. doi: 10.1016/s0165-0173(97)00035-0. Brain Res Brain Res Rev. 1998. PMID: 9651544 Review. - Disruption of hippocampal development in vivo by CR-50 mAb against reelin.
Nakajima K, Mikoshiba K, Miyata T, Kudo C, Ogawa M. Nakajima K, et al. Proc Natl Acad Sci U S A. 1997 Jul 22;94(15):8196-201. doi: 10.1073/pnas.94.15.8196. Proc Natl Acad Sci U S A. 1997. PMID: 9223338 Free PMC article. - Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody.
Utsunomiya-Tate N, Kubo K, Tate S, Kainosho M, Katayama E, Nakajima K, Mikoshiba K. Utsunomiya-Tate N, et al. Proc Natl Acad Sci U S A. 2000 Aug 15;97(17):9729-34. doi: 10.1073/pnas.160272497. Proc Natl Acad Sci U S A. 2000. PMID: 10920200 Free PMC article. - A truncated Reelin protein is produced but not secreted in the 'Orleans' reeler mutation (Reln[rl-Orl]).
de Bergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet AM, Miyata T, Ogawa M, Mikoshiba K. de Bergeyck V, et al. Brain Res Mol Brain Res. 1997 Oct 15;50(1-2):85-90. doi: 10.1016/s0169-328x(97)00166-6. Brain Res Mol Brain Res. 1997. PMID: 9406921 - [Cytoarchitectonic abnormality in the facial nucleus of the reeler mouse].
Terashima T, Setsu T, Kikkawa S, Ikeda Y. Terashima T, et al. Kaibogaku Zasshi. 1999 Aug;74(4):411-20. Kaibogaku Zasshi. 1999. PMID: 10496086 Review. Japanese.
Cited by
- New Insights into Reelin-Mediated Signaling Pathways.
Lee GH, D'Arcangelo G. Lee GH, et al. Front Cell Neurosci. 2016 May 9;10:122. doi: 10.3389/fncel.2016.00122. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27242434 Free PMC article. Review. - Contributions of VLDLR and LRP8 in the establishment of retinogeniculate projections.
Su J, Klemm MA, Josephson AM, Fox MA. Su J, et al. Neural Dev. 2013 Jun 13;8:11. doi: 10.1186/1749-8104-8-11. Neural Dev. 2013. PMID: 23758727 Free PMC article. - Bmp7 regulates the survival, proliferation, and neurogenic properties of neural progenitor cells during corticogenesis in the mouse.
Segklia A, Seuntjens E, Elkouris M, Tsalavos S, Stappers E, Mitsiadis TA, Huylebroeck D, Remboutsika E, Graf D. Segklia A, et al. PLoS One. 2012;7(3):e34088. doi: 10.1371/journal.pone.0034088. Epub 2012 Mar 26. PLoS One. 2012. PMID: 22461901 Free PMC article. - Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth.
Jossin Y, Goffinet AM. Jossin Y, et al. Mol Cell Biol. 2007 Oct;27(20):7113-24. doi: 10.1128/MCB.00928-07. Epub 2007 Aug 13. Mol Cell Biol. 2007. PMID: 17698586 Free PMC article. - Regulation of cortical neuron migration by the Reelin signaling pathway.
Honda T, Kobayashi K, Mikoshiba K, Nakajima K. Honda T, et al. Neurochem Res. 2011 Jul;36(7):1270-9. doi: 10.1007/s11064-011-0407-4. Epub 2011 Jan 21. Neurochem Res. 2011. PMID: 21253854 Review.
References
- Bar I, Lambert De Rouvroit C, Royaux I, Krizman DB, Dernoncourt C, Ruelle D, Beckers MC, Goffinet AM. A YAC contig containing the reeler locus with preliminary characterization of candidate gene fragments. Genomics. 1995;26:543–549. - PubMed
- Caviness VSJ. Time of origin in the hippocampus and dentate gyrus of normal and reeler mice: an autoradiographic analysis. J Comp Neurol. 1973;151:113–120. - PubMed
- Caviness VSJ, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. - PubMed
- Caviness VSJ, Crandall JE, Edwards MA. The reeler malformation. Implications for neocortical histogenesis. In: Peters A, Jones EG, editors. Cerebral cortex, Vol 7, Development and maturation of cerebral cortex. Plenum; New York: 1988. pp. 59–89.
- Curran T, D’Arcangelo G, Goffinet AM. reeler gene discrepancies. Nat Genet. 1995;11:12–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials