Activation of the CED3/ICE-related protease CPP32 in cerebellar granule neurons undergoing apoptosis but not necrosis - PubMed (original) (raw)
Comparative Study
Activation of the CED3/ICE-related protease CPP32 in cerebellar granule neurons undergoing apoptosis but not necrosis
R C Armstrong et al. J Neurosci. 1997.
Abstract
Neuronal apoptosis occurs during nervous system development and after pathological insults to the adult nervous system. Inhibition of CED3/ICE-related proteases has been shown to inhibit neuronal apoptosis in vitro and in vivo, indicating a role for these cysteine proteases in neuronal apoptosis. We have studied the activation of the CED3/ICE-related protease CPP32 in two in vitro models of mouse cerebellar granule neuronal cell death: K+/serum deprivation-induced apoptosis and glutamate-induced necrosis. Pretreatment of granule neurons with a selective, irreversible inhibitor of CED3/ICE family proteases, ZVAD-fluoromethylketone, specifically inhibited granule neuron apoptosis but not necrosis, indicating a selective role for CED3/ICE proteases in granule neuron apoptosis. Extracts prepared from apoptotic, but not necrotic, granule neurons contained a protease activity that cleaved the CPP32 substrate Ac-DEVD-aminomethylcoumarin. Induction of the protease activity was prevented by inhibitors of RNA or protein synthesis or by the CED3/ICE protease inhibitor. Affinity labeling of the protease activity with an irreversible CED3/ICE protease inhibitor, ZVK(biotin)D-fluoromethylketone, identified two putative protease subunits, p20 and p18, that were present in apoptotic but not necrotic granule neuron extracts. Western blotting with antibodies to the C terminus of the large subunit of mouse CPP32 (anti-CPP32) identified p20 and p18 as processed subunits of the CPP32 proenzyme. Anti-CPP32 specifically inhibited the DEVD-amc cleaving activity, verifying the presence of active CPP32 protease in the apoptotic granule neuron extracts. Western blotting demonstrated that the CPP32 proenzyme was expressed in granule neurons before induction of apoptosis. These results demonstrate that the CED3/ICE homolog CPP32 is processed and activated during cerebellar granule neuron apoptosis. CPP32 activation requires macromolecular synthesis and CED3/ICE protease activity. The lack of CPP32 activation during granule neuron necrosis suggests that proteolytic processing and activation of CED3/ICE proteases are specific biochemical markers of apoptosis.
Figures
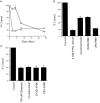
Fig. 1.
Cerebellar granule neuron cell death induced by withdrawal of K+/serum or exposure to glutamate.A, Time course of loss of viability of granule neurons after K+/serum deprivation (open diamonds) or exposure to 300 μ
m
glutamate (open squares). B, Viability of granule neurons 24 hr after K+/serum deprivation (5 m
m
K+/No Serum) without or with cycloheximide (Cycloheximide) (10 μg/ml), ZVAD-fmk (ZVAD-FMK) (50 μ
m
), or ZFA-fmk (ZFA-FMK) (50 μ
m
).C, Viability of granule neurons 2 hr after exposure to glutamate (300 μ
m
) without or with cycloheximide (10 μg/ml), ZVAD-fmk (50 μ
m
), or ZFA-fmk (50 μ
m
). Cell viability was measured using MTT. Values represent the mean ± SEM of triplicate cultures run in parallel and are expressed as percentage of untreated control cultures.
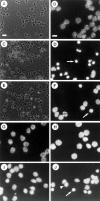
Fig. 2.
Phase-contrast (A, C, E) and Hoechst 33342 immunofluorescence (B, D, F–J) microscopy of cerebellar granule neurons after K+/serum withdrawal (A–H) or exposure to 300 μ
m
glutamate (I, J). Phase-contrast of control untreated neurons (A) or neurons 24 hr after high K+/serum withdrawal in the absence (C) or presence (E) of 50 μ
m
ZVAD-fmk. Note highly refractile, condensed cell bodies (∼2–3 μ
m
diameter) of apoptotic neurons in_C_ and the relative reduction in their proportion in_E_. Hoechst-labeled nuclei of control, untreated granule neurons (B), or neurons after 8 hr of K+/serum withdrawal in the absence (D) or presence (F) of 50 μ
m
ZVAD-fmk.Arrow in D marks a highly condensed, apoptotic nucleus. Arrow in F marks a nucleus that is less well protected by ZVAD-fmk than the surrounding nuclei. Hoechst-labeled nuclei of cells 24 hr after K+/serum withdrawal in the presence of either 10 μg/ml cycloheximide (G) or 1 μg/ml actinomycin D (H). Note normal size and morphology of nuclei in_G_ and H. Hoechst-labeled nuclei in cells 2 (I) or 8 hr (J) after exposure to 300 μ
m
glutamate. Note that nuclei in_I_ look normal in size and appearance of nucleoli.Arrow in J marks a nucleus that appears slightly shrunken compared with the normal-sized nucleus indicated by the arrowhead. Scale bars: A, 25 μ
m
; B, 10 μ
m
.
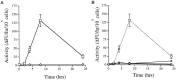
Fig. 3.
Induction of a Ac-DEVD-amc cleaving protease activity after K+/serum deprivation but not after exposure to glutamate. A, Time course of induction of Ac-DEVD-amc cleaving activity in extracts of granule neurons after K+/serum withdrawal (open squares) or exposure to 3 m
m
glutamate (open diamonds).B, Time course of induction of Ac-DEVD-amc cleaving activity in extracts of granule neurons after K+/serum deprivation in the absence (open squares with dotted line; data replotted from A) or in the presence of cycloheximide (10 μg/ml; open diamonds), actinomycin D (1 μg/ml; open circles), or ZVAD-fmk (50 μ
m
; open triangle). Values represent the mean ± SEM of triplicate reactions run in parallel and are expressed as the change in fluorescence units per hour per 105 cells.
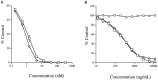
Fig. 4.
Inhibition by Ac-DEVD-aldehyde (A) and affinity-purified anti-CPP32 p20 (B) of the Ac-DEVD-amc cleaving activity present in extracts of granule neurons 8 hr after K+/serum deprivation. A, Dose–response of inhibition by Ac-DEVD-aldehyde of granule neuron protease activity (open squares) or recombinant human CPP32 protease (open diamonds). B, Dose–response of inhibition by anti-CPP32p20Pep of granule neuron protease activity (open squares), recombinant human CPP32 protease (open diamonds), or recombinant mouse ICE protease (open circles). Values represent the mean ± SEM of triplicate reactions run in parallel and are expressed as percentage of activity in uninhibited, control reactions.
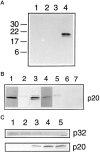
Fig. 5.
The CPP32 proenzyme is cleaved to generate p20/p18 subunits. A, Affinity labeling with ZVK(biotin)D-fmk of protease subunits in extracts of granule neurons before (lanes 1 and 2) or 8 hr after (_lanes 3_and 4) K+/serum withdrawal either in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of ZVK(biotin)D-fmk. Two proteins of _M_r 20 and 18 kDa are labeled in the 8 hr apoptotic extract. B, Western blotting with affinity-purified anti-CPP32p20Pep (lanes 1–3, 5–7) or with the anti-CPP32RP antiserum (lane 4). Lane 1: recombinant mouse CPP32; lane 2: extracts of unstimulated granule neurons; lanes 3 and 4: extracts of granule neurons 8 hr after K+/serum deprivation;lane 5: extracts of granule neurons 8 hr after K+/serum deprivation in the presence of 50 μ
m
ZVAD-fmk; lanes 6 and 7: extracts of granule neurons 2 hr (lane 6) or 8 hr (lane 7) after exposure to 300 μ
m
glutamate. C, Time course of disappearance of p32 proenzyme and appearance of p20 subunits before (lane 1) and at 2 hr (lane 2), 4 hr (lane 3), 6 hr (lane 4), and 8 hr (lane 5) after K+/serum withdrawal. Top panel was blotted with the anti-CPP32RP antiserum; bottom panel was blotted with the affinity-purified anti-CPP32p20Pep.
Similar articles
- Activation of CPP32 during apoptosis of neurons and astrocytes.
Keane RW, Srinivasan A, Foster LM, Testa MP, Ord T, Nonner D, Wang HG, Reed JC, Bredesen DE, Kayalar C. Keane RW, et al. J Neurosci Res. 1997 Apr 15;48(2):168-80. doi: 10.1002/(sici)1097-4547(19970415)48:2<168::aid-jnr9>3.0.co;2-a. J Neurosci Res. 1997. PMID: 9130145 - Processing/activation of CPP32-like proteases is involved in transforming growth factor beta1-induced apoptosis in rat hepatocytes.
Inayat-Hussain SH, Couet C, Cohen GM, Cain K. Inayat-Hussain SH, et al. Hepatology. 1997 Jun;25(6):1516-26. doi: 10.1002/hep.510250634. Hepatology. 1997. PMID: 9185777 - Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons.
Du Y, Bales KR, Dodel RC, Hamilton-Byrd E, Horn JW, Czilli DL, Simmons LK, Ni B, Paul SM. Du Y, et al. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11657-62. doi: 10.1073/pnas.94.21.11657. Proc Natl Acad Sci U S A. 1997. PMID: 9326666 Free PMC article. - The apoptotic cysteine protease CPP32.
Kumar S. Kumar S. Int J Biochem Cell Biol. 1997 Mar;29(3):393-6. doi: 10.1016/s1357-2725(96)00146-x. Int J Biochem Cell Biol. 1997. PMID: 9202418 Review.
Cited by
- Full length Bid is sufficient to induce apoptosis of cultured rat hippocampal neurons.
König HG, Rehm M, Gudorf D, Krajewski S, Gross A, Ward MW, Prehn JH. König HG, et al. BMC Cell Biol. 2007 Feb 27;8:7. doi: 10.1186/1471-2121-8-7. BMC Cell Biol. 2007. PMID: 17326836 Free PMC article. - Bone morphogenetic protein-6 promotes cerebellar granule neurons survival by activation of the MEK/ERK/CREB pathway.
Barneda-Zahonero B, Miñano-Molina A, Badiola N, Fadó R, Xifró X, Saura CA, Rodríguez-Alvarez J. Barneda-Zahonero B, et al. Mol Biol Cell. 2009 Dec;20(24):5051-63. doi: 10.1091/mbc.e09-05-0424. Mol Biol Cell. 2009. PMID: 19846661 Free PMC article. - Reperfusion, not simulated ischemia, initiates intrinsic apoptosis injury in chick cardiomyocytes.
Vanden Hoek TL, Qin Y, Wojcik K, Li CQ, Shao ZH, Anderson T, Becker LB, Hamann KJ. Vanden Hoek TL, et al. Am J Physiol Heart Circ Physiol. 2003 Jan;284(1):H141-50. doi: 10.1152/ajpheart.00132.2002. Epub 2002 Oct 10. Am J Physiol Heart Circ Physiol. 2003. PMID: 12388298 Free PMC article. - Hippocampal poly(ADP-Ribose) polymerase 1 and caspase 3 activation in neonatal bornavirus infection.
Williams BL, Hornig M, Yaddanapudi K, Lipkin WI. Williams BL, et al. J Virol. 2008 Feb;82(4):1748-58. doi: 10.1128/JVI.02014-07. Epub 2007 Dec 5. J Virol. 2008. PMID: 18057239 Free PMC article. - Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3.
Hongisto V, Smeds N, Brecht S, Herdegen T, Courtney MJ, Coffey ET. Hongisto V, et al. Mol Cell Biol. 2003 Sep;23(17):6027-36. doi: 10.1128/MCB.23.17.6027-6036.2003. Mol Cell Biol. 2003. PMID: 12917327 Free PMC article.
References
- Allsopp TE, Wyatt S, Patterson HF, Davies AM. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor dependent neurons from apoptosis. Neuron. 1993;7:295–307. - PubMed
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
- Armstrong RC, Aja T, Xiang J, Gaur S, Krebs JF, Hoang K, Bai X, Korsmeyer SJ, Karanewsky DS, Fritz LC, Tomaselli KJ. Fas-induced activation of the cell death related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Cell Biol. 1996;271:16850–16855. - PubMed
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials