A developmental gene (Tolloid/BMP-1) is regulated in Aplysia neurons by treatments that induce long-term sensitization - PubMed (original) (raw)
Comparative Study
A developmental gene (Tolloid/BMP-1) is regulated in Aplysia neurons by treatments that induce long-term sensitization
Q R Liu et al. J Neurosci. 1997.
Abstract
Long-term sensitization training, or procedures that mimic the training, produces long-term facilitation of sensory-motor neuron synapses in Aplysia. The long-term effects of these procedures require mRNA and protein synthesis (Montarolo et al., 1986; Castellucci et al., 1989). Using the techniques of differential display reverse transcription PCR (DDRT-PCR) and ribonuclease protection assays (RPA), we identified a cDNA whose mRNA level was increased significantly in sensory neurons by treatments of isolated pleural-pedal ganglia with serotonin for 1.5 hr or by long-term behavioral training of Aplysia. The effects of serotonin and behavioral training on this mRNA were mimicked by treatments that elevate cAMP. The aplysia mRNA increased by serotonin and behavioral training was 41-45% identical to a developmentally regulated gene family which includes Drosophila tolloid and human bone morphogenetic protein-1 (BMP-1). Both tolloid and BMP-1 encode metalloproteases that might activate TGF-beta (transforming growth factor beta)-like molecules or process procollagens. Aplysia tolloid/BMP-1-like protein (apTBL-1) might regulate the morphology and efficacy of synaptic connections between sensory and motor neurons, which are associated with long-term sensitization.
Figures
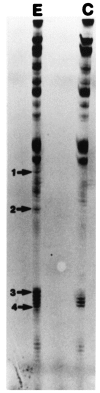
Fig. 1.
Differential display reverse transcription-PCR (DDRT-PCR). Total RNAs extracted from pleural-pedal ganglia treated with 5 μ
m
5-HT for 1.5 hr (E) or without treatment (C) were differentially displayed with an anchored oligo-dT (T12MG) and five arbitrary 10-mers (AGCCAGCGAA, GACCGCTTGT, AGGTGACCGT, GGTACTCCAC, and GTTGCGATCC). The bands (E) that appeared to be affected by 5-HT treatments are marked by arrows and_numbers_.
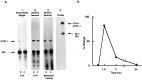
Fig. 2.
Ribonuclease protection assays of clone 2.A1, Effects of 5-HT on pleural-pedal ganglia. Experimental pleural-pedal ganglia were treated with 5 μ
m
5-HT for 1.5 hr, whereas matched contralateral control ganglia were untreated. Total RNAs (2 μg) from experimental (E) or control (C) pleural-pedal ganglia were hybridized with riboprobes of clone 2 and HSC70 (heat shock cognate protein).A2, Effects of 5-HT on sensory neurons. Total RNAs were extracted from sensory neurons of pleural-pedal ganglia treated with 5 μ
m
5-HT for 1.5 hr (E) or without treatment (C). A3, Effects of behavioral training on sensory neurons. Total RNAs were extracted from sensory neurons of pleural-pedal ganglia from the stimulated side (E) or unstimulated side (C) of animals. The size and purity of the probes are shown in A4.B, The time course of clone 2 mRNA change. Pleural-pedal ganglia were treated with 5 μ
m
5-HT for 0.75 and 1.5 hr, and sensory clusters were isolated and processed for RPA as described in Materials and Methods. After 1.5 hr, 5-HT was removed by washing with BFSW and ganglia were kept in BFSW. At 1.5 and 22.5 hr after removing 5-HT (time 3 and 24 hr), sensory clusters were isolated and processed for RPA.
Fig. 3.
Nucleotide and deduced amino acid sequences of apTBL-1 cDNA. apTBL-1 cDNA contains two possible translation initiation methionines (circled). The deduced amino acid sequence encodes a potential signal peptide at the N terminus (underlined with broken lines). Also, the deduced amino acid sequence contains the sequence homologous to the crayfish astacin family of metalloproteases (boxed), two 40-amino-acid repeats with EGF-like sequences (thick underlines), and seven potential glycosylation sites (thin underlined). The four cysteine residues for each CUB (complement subcomponents C1r/C1s, Uegf, BMP-1) repeat are enclosed in stippled or open boxes for alternate CUB repeats. The poly[A] signal sequences and RNA destabilization signal sequences are underlined with thin dashed lines. The nucleotide sequence of apTBL-1 cDNA has been submitted to GenBank under accession number U57369.
Fig. 4.
Distribution of apTBL-1 in various tissues of_Aplysia_. Total RNA (2.5 μg for pleural sensory neurons, 4 μg for other tissues) from Aplysia tissues was isolated and analyzed by RPA using the 32P-labeled apTBL-1 riboprobe discussed in Materials and Methods. The sources of RNA were as follows: PD, pedal ganglia;SN, pleural sensory neurons; CNS, central nervous system; GL, gill; HT, heart;KN, kidney; BW, body wall;HP, hepatopancreas; PN, penis;OT, ovotestis.
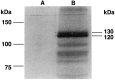
Fig. 5.
In vitro translation of apTBL-1.In vitro translation products with (B) or without (A) capped cRNA of full-length apTBL-1 cDNA in the presence of [35S]methionine. The Perfect Protein Marker (Novagen) was used as a molecular weight marker.
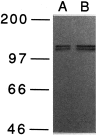
Fig. 6.
Immunoblot analysis of apTBL-1 proteins in pleural-pedal ganglia. Pleural-pedal ganglia of Aplysia californica were isolated and homogenized in 20 m
m
Tris-HCl, pH 7.5, containing 1 μ
m
leupeptin, 1 μ
m
chymostatin, 1 μ
m
pepstatin, 1 μ
m
bestatin, 5 m
m
EGTA, 5 m
m
EDTA, and 1 m
m
PMSF. The homogenate was centrifuged at 800 × g for 5 min. Immunoblot of the supernatant was performed as described in Materials and Methods. Ten micrograms of total protein (A) and 20 μg of total protein (B) were used for SDS-PAGE. Prestained molecular weight markers (Amersham)—myosin (_M_r = 200,000), phosphorylase b (_M_r= 97,400), bovine serum albumin (_M_r = 66,000), and ovalbumin (_M_r = 46,000)—were used.
Fig. 7.
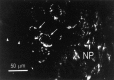
Immunolocalization of apTBL-1 in the pleural ganglion. The antibody directed against apTBL-1 protein produced punctuate staining in numerous cell bodies throughout the pleural ganglion, including sensory neurons (arrows). However, not all neurons in the sensory cluster were labeled (arrowheads). There was also staining in the neuropil (NP) and processes passing through the neuropil. In these relatively thick sections, it was not possible to identify stained structures in the neuropil.
Fig. 8.
Comparison of the domain structures of tolloid/BMP-1-like proteins. The tolloid/BMP-1 gene family includes Aplysia TBL-1, Drosophila tolloid (Shimell et al., 1991),tolloid-related-1 (Nguyen et al., 1994), mouse BMP-1 (Fukagawa et al., 1994), human BMP-1 (Wozney et al., 1988), sea urchin BMP-1 (Hwang et al., 1994), and Xenopus BMP-1 (Maeno et al., 1993). The potential signal peptide is represented by a_black box_, and propeptides are represented by_hatched boxes_. The metalloprotease domain, CUB repeats, and EGF-like repeats are marked accordingly. The C-terminal nonhomologous sequences are represented by open boxes. The group of apTBL-1, tolloid,tolloid-related-1, and muBMP-1 contains five CUB repeats and two EGF-like repeats, and the group of huBMP-1, suBMP-1, and xeBMP-1 contains three CUB repeats and one EGF-like repeat. The length of the signal peptide and propeptide at the N terminus is different among the members of the family.
Fig. 9.
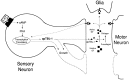
Model of possible roles of apTBL-1 in long-term presynaptic facilitation. A sensory neuron, motor neuron, and glial cell are represented schematically. The growth processes of sensory neurons and motor neurons are drawn with dotted lines. 5-HT increases the transcription of the apTBL-1 gene. apTBL-1 protein might remain in the cytoplasm by alternative translation and might play a role as a protease to modify the cytoskeleton structure in the growth process within the sensory neuron. apTBL-1 also might be secreted to modify the extracellular matrix (procollagen) or activate TGF-β-like growth factors. The activated growth factors could bind to Ser/Thr kinase receptors and trigger the signal transduction cascade, leading to the regulation of cell growth. The activated growth factors also might modify the motor neurons to complement the morphological changes in the sensory neurons, or they might activate glial cells to secrete extracellular matrix components that might then stabilize the morphological changes. Some of the same events elicited by the activation of TGF-β also could be caused by modification of the extracellular matrix component collagen.
Similar articles
- Identification of specific mRNAs affected by treatments producing long-term facilitation in Aplysia.
Zwartjes RE, West H, Hattar S, Ren X, Noel F, Nuñez-Regueiro M, MacPhee K, Homayouni R, Crow MT, Byrne JH, Eskin A. Zwartjes RE, et al. Learn Mem. 1998 Mar-Apr;4(6):478-95. doi: 10.1101/lm.4.6.478. Learn Mem. 1998. PMID: 10701873 - The two regulatory subunits of aplysia cAMP-dependent protein kinase mediate distinct functions in producing synaptic plasticity.
Liu J, Hu JY, Schacher S, Schwartz JH. Liu J, et al. J Neurosci. 2004 Mar 10;24(10):2465-74. doi: 10.1523/JNEUROSCI.4331-03.2004. J Neurosci. 2004. PMID: 15014122 Free PMC article. - Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia.
Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Hegde AN, et al. Cell. 1997 Apr 4;89(1):115-26. doi: 10.1016/s0092-8674(00)80188-9. Cell. 1997. PMID: 9094720 - Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change.
Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Bartsch D, et al. Cell. 1995 Dec 15;83(6):979-92. doi: 10.1016/0092-8674(95)90213-9. Cell. 1995. PMID: 8521521 - Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.
Glanzman DL. Glanzman DL. J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
Cited by
- Transcriptome analysis and identification of regulators for long-term plasticity in Aplysia kurodai.
Lee YS, Choi SL, Kim TH, Lee JA, Kim HK, Kim H, Jang DJ, Lee JJ, Lee S, Sin GS, Kim CB, Suzuki Y, Sugano S, Kubo T, Moroz LL, Kandel ER, Bhak J, Kaang BK. Lee YS, et al. Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18602-7. doi: 10.1073/pnas.0808893105. Epub 2008 Nov 18. Proc Natl Acad Sci U S A. 2008. PMID: 19017802 Free PMC article. - Registered Report: Transcriptional Analysis of Savings Memory Suggests Forgetting is Due to Retrieval Failure.
Rosiles T, Nguyen M, Duron M, Garcia A, Garcia G, Gordon H, Juarez L, Calin-Jageman IE, Calin-Jageman RJ. Rosiles T, et al. eNeuro. 2020 Nov 12;7(6):ENEURO.0313-19.2020. doi: 10.1523/ENEURO.0313-19.2020. Print 2020 Nov/Dec. eNeuro. 2020. PMID: 32928882 Free PMC article. - The ELAV family of RNA-binding proteins in synaptic plasticity and long-term memory.
Mirisis AA, Carew TJ. Mirisis AA, et al. Neurobiol Learn Mem. 2019 May;161:143-148. doi: 10.1016/j.nlm.2019.04.007. Epub 2019 Apr 15. Neurobiol Learn Mem. 2019. PMID: 30998973 Free PMC article. Review. - Binding of serotonin to receptors at multiple sites is required for structural plasticity accompanying long-term facilitation of Aplysia sensorimotor synapses.
Sun ZY, Schacher S. Sun ZY, et al. J Neurosci. 1998 Jun 1;18(11):3991-4000. doi: 10.1523/JNEUROSCI.18-11-03991.1998. J Neurosci. 1998. PMID: 9592080 Free PMC article. - Immediate and persistent transcriptional correlates of long-term sensitization training at different CNS loci in Aplysia californica.
Herdegen S, Conte C, Kamal S, Calin-Jageman RJ, Calin-Jageman IE. Herdegen S, et al. PLoS One. 2014 Dec 8;9(12):e114481. doi: 10.1371/journal.pone.0114481. eCollection 2014. PLoS One. 2014. PMID: 25486125 Free PMC article.
References
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1–20. - PubMed
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
- Appella E, Weber IT, Blasi F. Structure and function of epidermal growth factor-like regions in proteins. FEBS Lett. 1988;231:1–4. - PubMed
- Bailey CH, Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983;220:91–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous