Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat - PubMed (original) (raw)
Comparative Study
Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat
V Bernard et al. J Neurosci. 1997.
Abstract
Glutamate released in the basal ganglia is involved in the expression of clinical symptoms of neurodegenerative diseases like Parkinson's or Huntington's. Neostriatal neurons are the targets of glutamatergic inputs derived from the cortex and the thalamus acting via AMPA-type as well as other glutamate receptors. To determine the location of subunits of the AMPA subclass of glutamate receptors (GluR) in the rat neostriatum, we applied multiple immunocytochemical techniques using anti-peptide antibodies against the GluR1, GluR2/3, and GluR4 subunits at both the light and electron microscopic levels. All medium spiny efferent neurons, some of which were identified as striatonigral neurons, displayed immunoreactivity for GluR1 and GluR2/3 subunits. Double immunofluorescence revealed that at least 70-90% of parvalbumin-immunopositive GABAergic interneurons were immunoreactive for each of GluR1, GluR2/3, or GluR4 subunits and that at least 40% of choline acetyltransferase-immunopositive cholinergic interneurons were immunopositive for GluR1 or GluR4 subunits. The majority of nitric oxide synthase-immunopositive neurons had no detectable immunoreactivity for any of the AMPA receptor subunits. Electron microscopic analysis confirmed the presence of immunoreactivity for GluR1 and GluR2/3 in the perikarya of spiny neurons and interneurons and GluR4 in perikarya of interneurons only. GluR1 and GluR2/3 subunits were detected in dendrites and spines. A significant population of extrasynaptic receptors was revealed by pre-embedding immunogold labeling along the plasma membranes of perikarya, dendrites, and spines. Receptors were concentrated in the postsynaptic membrane specialization of asymmetrical synapses, as revealed by the postembedding immunogold method. Quantitative analysis demonstrated that immunoreactivity for the GluR1 and GluR2/3 subunits is higher at the periphery than at the middle of the postsynaptic membrane specialization. Our results demonstrate that AMPA receptor subunits are distributed widely and heterogeneously among striatal neurons and are concentrated on the postsynaptic membrane of asymmetrical synaptic specializations, although extrasynaptic receptors are also present.
Figures
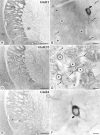
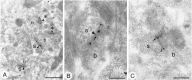
Fig. 1.
Immunohistochemical detection of GluR1 (A, B), GluR2/3 (C, D), and GluR4 (E, F) in the striatopallidal complex by the peroxidase method at the light microscopic level. The neostriatum (ns) and globus pallidus (gp) display immunoreactivity for GluR1, GluR2/3, and GluR4 subunits of the AMPA receptor. A dense dendritic staining of the neuropil is present in the neostriatum for GluR1 (A) and GluR2/3 (C), but not for GluR4 (E). Strongly labeled cells are identified throughout the neostriatum with GluR1 and GluR4 (some indicated by_arrows_), but not with GluR2/3 antibodies. Immunoreactivity for GluR1 (B) and GluR2/3 (D) is present in perikarya (asterisks) and dendrites (arrows) of striatal neurons that have characteristic features of spiny neurons. Some aspiny neurons with indented nuclei display strong immunoreactivity for GluR1 (B) and GluR4 (F) (curved arrows). cx, Cortex. Scale bars: A, C, E, 0.5 mm; B, D, F, 10 μm.
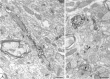
Fig. 2.
Identification of AMPA receptor subunits expressed by striatonigral neurons. Combination of the immunohistochemical detection of AMPA receptor subunits with the retrograde transport of WGA–HRP from the SN. Immunoreactivity for the receptor subunits was revealed by the immunoperoxidase method with DAB as the chromogen, and the transported WGA–HRP was revealed by using TMB-molybdate as the chromogen. Retrogradely labeled neurons containing granules of the peroxidase reaction product (some indicated by small arrows) also display immunoreactivity for GluR1 (A, asterisks) and GluR2/3 (B, asterisks), but not GluR4 (C, stars). Note the neurons (curved arrows in each micrograph) that possess characteristics of interneurons. Scale bars, 10 μm.
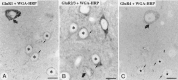
Fig. 3.
Identification of AMPA receptor subunits expressed by GABAergic interneurons using a double-immunofluorescence method. GABAergic interneurons were identified by using antibodies against parvalbumin (PV). PV-immunoreactive neurons (B, D, F) also display immunoreactivity for GluR1 (A), GluR2/3 (C), and GluR4 (E). Scale bars, 10 μm.
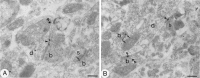
Fig. 4.
Subcellular localization of GluR1 in the neostriatum using the immunoperoxidase method. A, B, Reaction product is detected in dendrites (d) and in spines (s). The immunoreactive structures often are associated with axonal boutons (b) forming synapses (arrowheads), some of which are clearly asymmetrical. Scale bars, 0.5 μm.
Fig. 6.
Subcellular localization of GluR2/3 in the neostriatum using pre-embedding immunoperoxidase (B) and immunogold (A, C–E) methods. A, Part of an immunoreactive cell body in the neostriatum reacted by the immunogold method. The neuron possesses an indented nucleus (n) and large stacks of endoplasmic reticulum (er), identifying it as an interneuron. Immunoparticles are mainly in association with the cytoplasmic face of endoplasmic reticulum membrane, the cytoplasmic side of nuclear membrane (curved arrows), and on the internal surface of the plasma membrane (arrowheads). B, Immunoreactive dendrites (d) and spines (s) revealed by the immunoperoxidase method. Many of the immunoreactive spines are postsynaptic to boutons (b), forming asymmetrical synapses. C–E, Immunoreactive dendrites and spines reacted by the immunogold method. The immunoparticles are located mainly on the internal surface of the plasma membrane of spines and dendrites. Spines receive synapses from boutons forming asymmetrical synapses (arrowheads), and several of them have immunoparticles associated either with the body or the edges of the postsynaptic membrane specialization. Scale bars:A, B, D, 0.5 μm; C, E, 0.2 μm.
Fig. 7.
Subcellular localization of GluR4 immunoreactivity in the neostriatum using the pre-embedding immunoperoxidase (A, B) and immunogold (C, D) methods. GluR4 immunolabeling is detected in cell bodies (B, C) and dendrites (A, D). The immunoreactive cells have an indented nucleus (B) and a large volume of cytoplasm and often contain intranuclear inclusions (i), features that are characteristics of striatal interneurons. B, Aggregates of immunoperoxidase reaction product are seen in the cytoplasm (curved arrows) and in association with the nuclear membrane (open arrow). C, Immunoparticles are associated with the cytoplasmic face of membranes of the endoplasmic reticulum and the Golgi apparatus (g) and with the external side of nuclear membrane (small arrowheads). Dendrites that are immunolabeled for GluR4 are surrounded by boutons (b), many of which form asymmetrical synapses (A, D).D, Immunoparticles are associated with the edge of the postsynaptic density of the asymmetrical synapses (arrowheads). Scale bars: A, 0.5 μm;B, C, 1 μm; D, 0.2 μm.
Fig. 5.
Subcellular localization of GluR1 in the neostriatum using pre-embedding immunogold method with silver intensification. A, Immunopositive cell body with an indented nucleus (n) and large volume of cytoplasm, characteristic of striatal interneurons. The immunoparticles are associated with the cytoplasmic surface of the membrane of the endoplasmic reticulum (er) and, albeit to lesser extent, the Golgi apparatus (g). Immunoparticles also are located on the external side of the nuclear membrane (curved arrows) and on the internal side of the plasma membrane (arrowheads). B–D, Immunolabeled dendrites (d) and spines (s). The immunoparticles are associated mainly with the internal surface of the plasma membrane. In C and D, immunopositive dendrites are surrounded by numerous boutons (b), many of which make asymmetrical synaptic contacts with them, which is a characteristic of interneurons. Immunoparticles are associated with the extrasynaptic plasma membrane and several of the synapses (arrowheads), including axospinous synapses (s) (B, D). The immunoparticles often are localized at the edge of the synaptic specialization, although sometimes (in the spine in B and the _top_labeled synapse in C) the immunoparticles appear over the postsynaptic membrane specialization. Scale bars:A, 1 μm; B, 0.2 μm; C, D, 0.5 μm.
Fig. 8.
Subcellular localization of GluR1 immunoreactivity in the neostriatum revealed by the postembedding immunogold method with silver intensification. Dendrites (d) (A, B) and a spine (s) (A) are immunolabeled and receive asymmetrical synapses from boutons (b). The small immunoparticles are located mainly on the postsynaptic membrane both within the body of the synaptic specialization (arrowheads) and at its periphery (arrows). The two large particles (open arrowheads) are not attributable to immunolabeling but are a result of the silver intensification process. Scale bars, 0.2 μm.
Fig. 9.
Subcellular localization of GluR2/3 in the neostriatum using the postembedding immunogold method with silver intensification. Electron micrographs show spines (s) immunolabeled for GluR2/3. At low (A) and high (B, C) magnification, immunolabeled spines are seen in synaptic contact with boutons (b) forming asymmetrical synapses (arrowheads). Some postsynaptic profiles (asterisks) could be spines or small dendritic shafts. Immunoparticles occasionally form clusters and seem to be distributed throughout the postsynaptic membrane (arrowheads in_A_ and B) or are located at the periphery of the postsynaptic specialization (arrows in_C_). Scale bars: A, 0.5 μm; B, C, 0.2 μm.
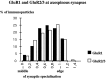
Fig. 10.
Distribution of immunoparticles for_GluR1_ and GluR2/3 subunits along the postsynaptic membrane specialization of axospinous synapses labeled by the postembedding immunogold method. A similar distribution of_GluR1_ and GluR2/3 subunits occurred at axospinous synapses [GluR1, n = 30 synapses (mean length ± SEM, 279 ± 9 nm), 97 immunoparticles; GluR2/3, n = 88 synapses (mean length ± SEM, 257 ± 19 nm), 410 immunoparticles]. Immunoparticles are concentrated at the periphery of the postsynaptic membrane specialization. Only synapses labeled by two or more particles are included in the analysis.
Similar articles
- Cellular localization of GluR1, GluR2/3 and GluR4 glutamate receptor subunits in neurons of the rat neostriatum.
Kwok KH, Tse YC, Wong RN, Yung KK. Kwok KH, et al. Brain Res. 1997 Dec 5;778(1):43-55. doi: 10.1016/s0006-8993(97)00950-5. Brain Res. 1997. PMID: 9462876 - Cellular localization of AMPA type glutamate receptor subunits in the basal ganglia of pigeons (Columba livia).
Laverghetta AV, Toledo CA, Veenman CL, Yamamoto K, Wang H, Reiner A. Laverghetta AV, et al. Brain Behav Evol. 2006;67(1):10-38. doi: 10.1159/000088856. Epub 2005 Oct 10. Brain Behav Evol. 2006. PMID: 16219996 - Parvalbumin-containing interneurons in rat hippocampus have an AMPA receptor profile suggestive of vulnerability to excitotoxicity.
Moga D, Hof PR, Vissavajjhala P, Moran TM, Morrison JH. Moga D, et al. J Chem Neuroanat. 2002 May;23(4):249-53. doi: 10.1016/s0891-0618(02)00012-1. J Chem Neuroanat. 2002. PMID: 12048108 Review. - Chemical anatomy of striatal interneurons in normal individuals and in patients with Huntington's disease.
Cicchetti F, Prensa L, Wu Y, Parent A. Cicchetti F, et al. Brain Res Brain Res Rev. 2000 Nov;34(1-2):80-101. doi: 10.1016/s0165-0173(00)00039-4. Brain Res Brain Res Rev. 2000. PMID: 11086188 Review.
Cited by
- Heterogeneous properties of central lateral and parafascicular thalamic synapses in the striatum.
Ellender TJ, Harwood J, Kosillo P, Capogna M, Bolam JP. Ellender TJ, et al. J Physiol. 2013 Jan 1;591(1):257-72. doi: 10.1113/jphysiol.2012.245233. Epub 2012 Oct 29. J Physiol. 2013. PMID: 23109111 Free PMC article. - Synaptic AMPA receptor composition in development, plasticity and disease.
Henley JM, Wilkinson KA. Henley JM, et al. Nat Rev Neurosci. 2016 Jun;17(6):337-50. doi: 10.1038/nrn.2016.37. Epub 2016 Apr 15. Nat Rev Neurosci. 2016. PMID: 27080385 Review. - Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity.
Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Sattler R, et al. J Neurosci. 2000 Jan 1;20(1):22-33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. J Neurosci. 2000. PMID: 10627577 Free PMC article. - Use of electron microscopy in the detection of adrenergic receptors.
Aoki C, Rodrigues S, Kurose H. Aoki C, et al. Methods Mol Biol. 2000;126:535-63. doi: 10.1385/1-59259-684-3:535. Methods Mol Biol. 2000. PMID: 10685434 Free PMC article. Review. No abstract available. - Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats.
Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Tang W, et al. J Neurochem. 2004 May;89(4):1021-33. doi: 10.1111/j.1471-4159.2004.02392.x. J Neurochem. 2004. PMID: 15140200 Free PMC article.
References
- Albin RL, Greenamyre JT. Alternative excitotoxic hypotheses. Neurology. 1992;42:733–738. - PubMed
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–374. - PubMed
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
- Baude A, Nusser Z, Molnar E, McIlhinney RAJ, Somogyi P. High-resolution immunogold localization of AMPA-type glutamate receptor subunits at synaptic and nonsynaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. - PubMed
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurones in the neostriatum of the rat. Neuroscience. 1994;62:707–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous