Cell surface amyloid beta-protein precursor colocalizes with beta 1 integrins at substrate contact sites in neural cells - PubMed (original) (raw)
Cell surface amyloid beta-protein precursor colocalizes with beta 1 integrins at substrate contact sites in neural cells
T Yamazaki et al. J Neurosci. 1997.
Abstract
Amyloid beta-protein (A beta), the principal constituent of the senile plaques seen in Alzheimer's disease (AD), is derived by proteolysis from the beta-amyloid precursor protein (beta PP). The distribution and trafficking of cell surface beta PP are of particular interest because some of these molecules are direct precursors of secreted A beta and because the localization of beta PP at the cell surface may be related directly to its physiological functions. Recently, we reported that, in cultured hippocampal neurons, cell surface beta PP is preferentially expressed on axons in a striking discontinuous pattern. In this study, we describe the colocalization of cell surface beta PP and integrins in primary cultured cells. In rat hippocampal neurons, cell surface beta PP was colocalized selectively with alpha 1 beta 1 and alpha 5 beta 1 integrin heterodimers at these characteristic segmental locations. In rat cortical astrocytes, both cell surface beta PP and beta 1 integrin were located at the cell periphery in the "spreading" stage shortly after plating. In "flattened" astrocytes cultured for several days, beta PP was found in punctate deposits called point contacts. In these sites, beta PP was colocalized with alpha 1 beta 1, but not with alpha 5 beta 1 integrin heterodimers, the latter of which were situated at focal contact sites. In both neurons and astrocytes examined after shearing, clathrin and alpha-adaptin were colocalized with beta PP on the surface that directly contacts the substratum. These results are consistent with the putative role of beta PP in cell adhesion and suggests that beta PP either interacts with selected integrins or shares similar cellular machinery to promote cell adhesion.
Figures
Fig. 1.
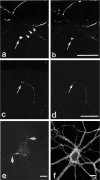
Immunocytochemical colocalization of cell surface βPP and integrins in cultured hippocampal neurons. A hippocampal neuron cultured for 14 d was double-labeled for β1 integrin (a) and cell surface βPP (5A3/1G7)(b). The axonal pattern from both immunostaining reactions was patchy and overlapped entirely. β1 integrin and βPP on the perikaryal surface cannot be compared clearly, because that region of the cell body is not within the plain of focus of the photomicrographs. α1 (c) and α5 (e) subunits of integrins also were colocalized with cell surface βPP (d, f) along neurites. Scale bars, 5 μm.
Fig. 2.
Immunocytochemical colocalization of cell surface βPP and integrins at growth cones. Shown are two examples of colocalization of cell surface βPP (a, c) at growth cones with β1 (b) and α5 (d) integrins in mature hippocampal neurons in culture, as seen by double-labeling. The patchy surface distribution of βPP is highlighted in a, where the _arrowheads_trace out the segment of axon devoid of surface βPP immunoreactivity. To examine cell substrate contact sites, we sheared neurons (see Materials and Methods) and stained them with anti-βPP antibodies (5A3/1G7)(e). βPP was localized on neurites (arrows) and on the cell body in a granular pattern. Neuronal cell adhesion molecule (NCAM) staining (f) showed a diffuse, rather than a patchy, pattern on neurites. Scale bars, 5 μm.
Fig. 5.
Immunocytochemical colocalization of cell surface βPP with clathrin and α-adaptin. a, A hippocampal neuron cultured for 10 d labeled with an anti-clathrin antibody (X22) showed a fine punctate staining pattern on neurites, but its distribution was not patchy and occurred predominantly in axons (compare with βPP shown in Fig. 1_b_). On the other hand, at substrate contact sites visualized in sheared neurons, βPP (antibody 207) (b) and clathrin (c) were specifically colocalized. Within the immunoreactive patches, there is a suggestion of fine granular staining. In sheared astrocytes, βPP (207) (d) also was tightly colocalized with α-adaptin, as demonstrated by antibody AP.6 (e). Scale bars, 5 μm.
Fig. 3.
Immunocytochemical colocalization of cell surface βPP and β1 integrin in sympathetic ganglion neurons cultured for 7 d on type 1 collagen- or laminin-coated glass coverslips in serum-free medium. On both type 1 collagen (a, b) and laminin (c, d), cell surface βPP (a, c) and β1 integrin (b, d) showed the characteristic segmental pattern and tight colocalization by double labeling. Scale bars, 10 μm.
Fig. 4.
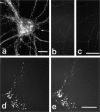
Immunocytochemical colocalization of cell surface βPP and integrins in type 1 astrocytes. Type 1 astrocytes were allowed to attach and spread on laminin or fibronectin for 3 hr (spreading stage) and then were labeled with βPP (5A3/1G7) (a) and β1 integrin (b) antibodies. Both molecules were located mainly at the periphery and in the middle of the spreading cells. In cells cultured for 3 d before fixation (“flattened” astrocytes), cell surface βPP (c) and β1 integrin (d) were now colocalized at point contact sites. More specifically, surface βPP (antibody 207)(e) was tightly colocalized with the α1 subunit of integrins (f) when examined in sheared astrocytes. In contrast, the α5 subunit of integrins was localized in focal contact sites, appearing as linear streaks in the sheared cells (h, arrows), and it did not colocalize with βPP (5A3/1G7) (g). At point contact sites, staining with βPP midregion (1G7/5A3) (i) and C-terminal (C7) (j) antibodies in sheared cells showed complete colocalization, suggesting that βPP at the substrate surface represents full-length molecules. Scale bars:a–h, 10 μm; i, j, 5 μm.
Similar articles
- Trafficking of cell surface beta-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons.
Yamazaki T, Selkoe DJ, Koo EH. Yamazaki T, et al. J Cell Biol. 1995 Apr;129(2):431-42. doi: 10.1083/jcb.129.2.431. J Cell Biol. 1995. PMID: 7721945 Free PMC article. - Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts.
Tawil N, Wilson P, Carbonetto S. Tawil N, et al. J Cell Biol. 1993 Jan;120(1):261-71. doi: 10.1083/jcb.120.1.261. J Cell Biol. 1993. PMID: 8416993 Free PMC article. - Characterization of neural cell adhesion sites: point contacts are the sites of interaction between integrins and the cytoskeleton in PC12 cells.
Arregui CO, Carbonetto S, McKerracher L. Arregui CO, et al. J Neurosci. 1994 Nov;14(11 Pt 2):6967-77. doi: 10.1523/JNEUROSCI.14-11-06967.1994. J Neurosci. 1994. PMID: 7965092 Free PMC article. - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - The molecular significance of amyloid beta-peptide for Alzheimer's disease.
Haass C. Haass C. Eur Arch Psychiatry Clin Neurosci. 1996;246(3):118-23. doi: 10.1007/BF02189111. Eur Arch Psychiatry Clin Neurosci. 1996. PMID: 8739395 Review.
Cited by
- Integrins as receptor targets for neurological disorders.
Wu X, Reddy DS. Wu X, et al. Pharmacol Ther. 2012 Apr;134(1):68-81. doi: 10.1016/j.pharmthera.2011.12.008. Epub 2011 Dec 30. Pharmacol Ther. 2012. PMID: 22233753 Free PMC article. Review. - Amyloid precursor protein processing and bioenergetics.
Wilkins HM, Swerdlow RH. Wilkins HM, et al. Brain Res Bull. 2017 Jul;133:71-79. doi: 10.1016/j.brainresbull.2016.08.009. Epub 2016 Aug 18. Brain Res Bull. 2017. PMID: 27545490 Free PMC article. Review. - Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance.
Sosa LJ, Bergman J, Estrada-Bernal A, Glorioso TJ, Kittelson JM, Pfenninger KH. Sosa LJ, et al. PLoS One. 2013 May 14;8(5):e64521. doi: 10.1371/journal.pone.0064521. Print 2013. PLoS One. 2013. PMID: 23691241 Free PMC article. - Huntingtin and Other Neurodegeneration-Associated Proteins in the Development of Intracellular Pathologies: Potential Target Search for Therapeutic Intervention.
Churkina Taran AS, Shakhov AS, Kotlobay AA, Alieva IB. Churkina Taran AS, et al. Int J Mol Sci. 2022 Dec 8;23(24):15533. doi: 10.3390/ijms232415533. Int J Mol Sci. 2022. PMID: 36555175 Free PMC article. Review. - The insect homologue of the amyloid precursor protein interacts with the heterotrimeric G protein Go alpha in an identified population of migratory neurons.
Swanson TL, Knittel LM, Coate TM, Farley SM, Snyder MA, Copenhaver PF. Swanson TL, et al. Dev Biol. 2005 Dec 1;288(1):160-78. doi: 10.1016/j.ydbio.2005.09.029. Epub 2005 Oct 17. Dev Biol. 2005. PMID: 16229831 Free PMC article.
References
- Avnur Z, Geiger B. Substrate-attached membranes of cultured cells isolation and characterization of ventral cell membranes and the associated cytoskeleton. J Mol Biol. 1981;153:361–379. - PubMed
- Breen KC, Bruce M, Anderton BH. Beta amyloid precursor protein mediates neuronal cell-cell and cell-surface adhesion. J Neurosci Res. 1991;28:90–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous