The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood - PubMed (original) (raw)
The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood
A Aiuti et al. J Exp Med. 1997.
Abstract
Hematopoietic progenitor cells migrate in vitro and in vivo towards a gradient of the chemotactic factor stromal cell-derived factor-1 (SDF-1) produced by stromal cells. This is the first chemoattractant reported for human CD34+ progenitor cells. Concentrations of SDF-1 that elicit chemotaxis also induce a transient elevation of cytoplasmic calcium in CD34+ cells. SDF-1-induced chemotaxis is inhibited by pertussis toxin, suggesting that its signaling in CD34+ cells is mediated by seven transmembrane receptors coupled to Gi proteins. CD34+ cells migrating to SDF-1 include cells with a more primitive (CD34+/CD38- or CD34+/DR-) phenotype as well as CD34+ cells phenotypically committed to the erythroid, lymphoid and myeloid lineages, including functional BFU-E, CFU-GM, and CFU-MIX progenitors. Chemotaxis of CD34+ cells in response to SDF-1 is increased by IL-3 in vitro and is lower in CD34+ progenitors from peripheral blood than in CD34+ progenitors from bone marrow, suggesting that an altered response to SDF-1 may be associated with CD34 progenitor mobilization.
Figures
Figure 4
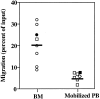
Mobilized PB CD34+ cells are less responsive to SDF-1 induced chemotaxis than their BM counterparts. PB samples were obtained from solid tumor patients treated in the hematological recovery phase of chemotherapy + G-CSF treatment (n = 6, squares), or on day 5 of G-CSF treatment alone (n = 1, diamond), as described in Materials and Methods. BM samples were obtained from normal donors (n = 6; circles) or from solid tumor patient in remission (n = 1; square). Closed circles represent one set of CD34+ cells purified from BM or PB samples obtained the same day of treatment from the same tumor patient treated in the hematological recovery phase of chemotherapy + G-CSF treatment. Symbols indicate the percentage of CD34+ cells migrating to SDF-1 (300 ng/ml) in a transendothelial assay. Each symbol represents one different preparation from a different donor. The horizontal bars indicate the average percentage of migration for the bone marrow and the mobilized peripheral blood CD34+cell samples, respectively. Data for PB CD34+ cells are the following: average 5.1% of input, SEM 0.58. Data for BM CD34+ cells are the following: average 20% of input, SEM 3.4; P <0.01. The proportion of cells migrating to control media in these transendothelial assays ranged from 0.05 to 0.5% percent of input, resulting in a low accuracy in detecting few cells (<1,000) by flow cytometry and a large variability of chemotactic indexes (CI). Therefore, no statistically significant differences were found between the CI of BM and PB CD34+ cells to SDF-1, although a tendency was observed for lower CI in PB CD34+ cells (median CI for BM CD34+, 48 [SEM 8]; for PB CD34+, 24 [SEM 16]).
Figure 1
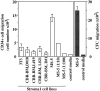
BM stromal cells produce a chemoattractant for human CD34+ progenitor cells. CD34+ cells from human cord blood (CB) (2.0 × 105/well) were assayed for their capacity to migrate in a Boyden chamber (5 μm bare filters), towards conditioned media from different BM stromal cell lines. The migratory population was evaluated phenotypically as the number of CD34+ cells that migrated (left, open bars), and functionally as the number of clonogenic progenitors (CFC) that migrated to the conditioned media (right, closed bars). Bars and columns indicate the duplicate and mean of one representative experiment out of three.
Figure 2
Stromal-derived SDF-1 is a chemoattractant for human and mouse hematopoietic progenitor cells. (A) Chemotaxis assay of cord blood (CB) CD34+ cells in response to various concentration of SDF-1 (10, 30, 100, 300, 1,000 ng/ml). Results represent the average and the range of three experiments performed in duplicates. Data are expressed as the percent of input cells that migrated. The percentage of migration of CB CD34+ to undiluted MS-5 supernatant in the same experiments was used as control and is shown as a bar diagram on the right (n = 3). (B) Chemotactic response to SDF-1 of clonogenic progenitors (CFC). The graph shows the number of CFU-GM, BFU-E, and CFU-MIX progenitors from human CB CD34+ cells that migrated to 300 ng/ml of SDF-1 or control media in a chemotaxis assay. (C) Transendothelial chemotaxis in vitro of human bone marrow (BM) or mobilized peripheral blood (PB) CD34+ cells in response to different concentration of SDF-1. Results show the average and the range of three experiments performed in duplicates. (D) Transendothelial chemotaxis of mobilized PB CD34+ cells in response to SDF-1 or to various chemoattractants and cytokines at the concentrations described in the Materials and Methods. (E) Transendothelial chemotaxis in vitro of the mouse progenitor cell lines FDCP-MIX and M1 in response to SDF-1. (F). In vivo delivery of SDF-1 into mouse spleens increases the seeding of intravenously transplanted FDCP-MIX cells in this organ. Experimental mice were injected intrasplenically with SDF-1 and control mice were injected with MIP-1α or with PBS and then transplanted i.v. with PKH-26-labeled FDCP-MIX cells, as described in Materials and Methods (three experimental mice and three control mice per experiment; three separate experiments performed). 3 h after the injection, mice were killed and 5 × 105 splenocytes plated in duplicate in a clonogenic assay for FDCP-MIX. After 72 h, the plates were counted on an inverted fluorescent microscope and scored for the number of fluorescent colonies. Results show the significant increase in FDCP-MIX clonogenic precursors per SDF-1 injected spleens relative to control injected spleens. Data shown are from three experiments: *P <0.005.
Figure 3
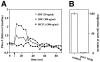
SDF-1 induces calcium fluxes in human CD34+ cells and the associated chemotaxis is pertussis toxin sensitive. (A) SDF-1 induces calcium fluxes in human CD34+cells. Human BM CD34+ cells were loaded with the calcium-sensitive dye Fluo-3. Changes in Fluo-3 emission in response to SDF-1 (30 ng/ml, open circles; 300 ng/ml, closed circles) or human MCP-1 (300 ng/ml, open squares) were monitored over time by flow cytometry. (B) The chemotaxis of human CD34+ to SDF-1 is pertussis toxin sensitive. The chemotactic response of human BM CD34+ cells to SDF-1 (300 ng/ml) after a 2-h incubation in 10% FCS IMDM with (100 ng/ml; pert toxin) or without pertussis toxin (control). Results shown are the mean (and duplicates) of the percentage of the migration of the control samples (which are considered as 100%). Actual percentage of migration in the control ranged from 18–25% of input. Data are representative of three separate experiments.
Figure 5
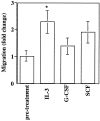
Cytokine modulation of CD34+ cell chemotaxis to SDF-1. CD34+ cells were incubated for 18 h in the presence of either 30 ng/ml IL-3, 50 ng/ml G-CSF or 100 ng/ml SCF and assayed for transendothelial chemotaxis in response to 300 ng/ml of SDF-1. Results show the mean fold change in the chemotactic response of CD34+ in three separate experiments performed in duplicate, with respect to chemotaxis before treatment (*P <0.05).
Similar articles
- Changes in the growth properties of CD34+, CD38- bone marrow progenitors during human fetal development.
Waller EK, Huang S, Terstappen L. Waller EK, et al. Blood. 1995 Jul 15;86(2):710-8. Blood. 1995. PMID: 7541673 - Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice.
Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, Guetta E, Barkai G, Nagler A, Lapidot T. Kollet O, et al. Blood. 2001 May 15;97(10):3283-91. doi: 10.1182/blood.v97.10.3283. Blood. 2001. PMID: 11342460 - Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1.
Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A, Lambris JD, Ratajczak MZ. Reca R, et al. Blood. 2003 May 15;101(10):3784-93. doi: 10.1182/blood-2002-10-3233. Epub 2003 Jan 2. Blood. 2003. PMID: 12511407
Cited by
- Emerging insights into epigenetics and hematopoietic stem cell trafficking in age-related hematological malignancies.
Xinyi Y, Vladimirovich RI, Beeraka NM, Satyavathi A, Kamble D, Nikolenko VN, Lakshmi AN, Basappa B, Reddy Y P, Fan R, Liu J. Xinyi Y, et al. Stem Cell Res Ther. 2024 Nov 6;15(1):401. doi: 10.1186/s13287-024-04008-4. Stem Cell Res Ther. 2024. PMID: 39506818 Free PMC article. Review. - Bone marrow mesenchymal stem cells overexpressing stromal cell- derived factor 1 aid in bone formation in osteoporotic mice.
Wang Y, Xiao Y, Yang X, He F, Hu J, Yang G, Wang W. Wang Y, et al. BMC Musculoskelet Disord. 2024 Nov 4;25(1):878. doi: 10.1186/s12891-024-07957-2. BMC Musculoskelet Disord. 2024. PMID: 39497150 Free PMC article. - Navigating uncharted waters: assessing the impact of the COVID-19 pandemic on hematopoietic stem cell transplantation: challenges and innovations.
Qureshi Z, Altaf F, Jamil A, Siddique R, Shah S. Qureshi Z, et al. Ann Med Surg (Lond). 2024 Aug 7;86(9):5416-5424. doi: 10.1097/MS9.0000000000002442. eCollection 2024 Sep. Ann Med Surg (Lond). 2024. PMID: 39239009 Free PMC article. Review. - Association of SDF-1-3' Gene A Variant with Diabetic Retinopathy in the Hungarian Population.
Ecsedy M, Kovacs I, Szigeti A, Horvath H, Lenart L, Recsan Z, Medveczki T, Nagy ZZ, Fekete A. Ecsedy M, et al. Int J Mol Sci. 2024 Jul 23;25(15):8036. doi: 10.3390/ijms25158036. Int J Mol Sci. 2024. PMID: 39125605 Free PMC article. - Donor and recipient hematopoietic stem and progenitor cells mobilization in liver transplantation patients.
Zhi Y, Qiu W, Tian G, Song S, Zhao W, Du X, Sun X, Chen Y, Huang H, Li J, Yu Y, Li M, Lv G. Zhi Y, et al. Stem Cell Res Ther. 2024 Jul 29;15(1):231. doi: 10.1186/s13287-024-03855-5. Stem Cell Res Ther. 2024. PMID: 39075608 Free PMC article.
References
- Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte–macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;1:1194–1198. - PubMed
- Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni AM. Circulation of CD34+hematopoietic stem cells in the peripheral blood of high-dose cyclophosphamide-treated patients: enhancement by intravenous recombinant human granulocyte-macrophage colonystimulating factor. Blood. 1989;6:1905–1914. - PubMed
- Sheridan WP, Begley CG, Juttner CA, Szer J, Bik L, To, Maher D, McGrath KM, Morstyn G, Fox RM. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–644. - PubMed
- Elias AD, Ayash L, Anderson KC, Hunt M, Wheeler C, Schwartz G, Tepler I, Mazanet R, Lynch C, Pap S, et al. Mobilization of peripheral blood progenitor cells by chemotherapy and granulocyte–macrophage colony-stimulating factor for hematologic support after high-dose intensification for breast cancer. Blood. 1992;79:3036–3044. - PubMed
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials