Retrograde transport and steady-state distribution of 125I-nerve growth factor in rat sympathetic neurons in compartmented cultures - PubMed (original) (raw)
Retrograde transport and steady-state distribution of 125I-nerve growth factor in rat sympathetic neurons in compartmented cultures
D R Ure et al. J Neurosci. 1997.
Abstract
We have used compartmented cultures of rat sympathetic neurons to quantitatively examine the retrograde transport of 125I-nerve growth factor (NGF) supplied to distal axons and to characterize the cellular events that maintain steady-state levels of NGF in cell bodies. In cultures allowed to reach steady-state 125I-NGF transport, cell bodies contained only 5-30% of the total neuron-associated 125I-NGF, whereas 70-95% remained associated with the distal axons. This was true over an 8 pM to 1.5 nM 125I-NGF concentration range, indicating that saturation of high affinity receptors could not account for the large fraction of 125I-NGF remaining in axons. Dissociation assays indicated that 85% of 125I-NGF associated with distal axons was surface-bound. At steady-state, only 2-25% of the distal axon-associated 125I-NGF was retrogradely transported each hour, with higher transport rates associated with younger cultures and lower 125I-NGF concentrations. The velocity of 125I-NGF retrograde transport was estimated at 10-20 mm/hr. However, as in a previous report, almost no 125I-NGF transport was observed during the first hour after 125I-NGF administration, indicating a significant lag between receptor binding and loading onto the retrograde transport system. During 125I-NGF transport through axons spanning an intermediate compartment in five-compartment cultures, little or no 125I-NGF was degraded or released from the axons. After transport, 125I-NGF was degraded with a half-life of 3 hr. In summary, although some cellular events promoted NGF accumulation in cell bodies, distal axons represented by far the principal site of NGF-receptor interaction at steady-state as a result of a low retrograde transport rate.
Figures
Fig. 1.
Compartmented culture designs. Illustrations of individual tracks (20 tracks per culture) are shown from three different designs of compartmented cultures used in the present study. Teflon septa separate compartments. Distal axon compartments, in which125I-NGF was always applied, were separated from cell body/proximal axon compartments by distances of the following:a, 1 mm; b, 6 mm; c, 8 mm. Illustrations are not to scale.
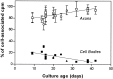
Fig. 2.
Relative distributions of cell-associated NGF. In three-compartment cultures of various ages, 0.2–40 ng/ml125I-NGF (8 p
m
to 1.5 n
m
) was supplied to distal axons for 15–24 hr, during which time some125I-NGF was retrogradely transported to cell bodies/proximal axons. Distal axons in some cultures additionally received ≥100-fold excess NGF for determination of nonspecific association/transport. After removing the culture medium and rinsing all compartments, we made cell extracts and quantified the radioactivity. Shown are relative proportions of 125I-NGF radioactivity associated with distal axons or accumulated in cell bodies/proximal axons, with nonspecific values subtracted. Concentrations of applied 125I-NGF are 0.2 ng/ml (circles); 3–20 ng/ml (squares); 40 ng/ml (triangles). Distal axon-associated125I-NGF is the sum of both side compartments. In each experiment two to four cultures were used per treatment group. Error bars ± SEM fall within symbols when not visible. Linear regressions were calculated from data from a total of 17 experiments.
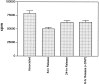
Fig. 3.
125I-NGF dissociation from distal axons. In 37-d-old three-compartment cultures, distal axons were supplied with 20 ng/ml 125I-NGF, alone or with ≥100-fold excess NGF for 10 hr, after which cultures from each of these two groups were split into three subsequent groups. (1) Distal axons were harvested immediately (6 sides); (2) Axonal 125I-NGF was chased with 0.4 μg/ml NGF for 6 hr and then for 18 hr at 37°C (6 sides), and the dissociated 125I-NGF was collected after each chase; (3) Axonal 125I-NGF was chased with 0.4 μg/ml NGF and 500 μ
m
dinitrophenol (1000× dilution) for 6 hr at 37°C (4 sides), and the dissociated 125I-NGF was collected. Specific values for 125I-NGF association and dissociation are shown (means ± SEM). Two other experiments gave similar results.
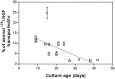
Fig. 4.
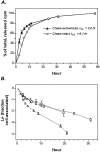
Rates of retrograde transport. Data are taken from experiments described in Figure 2. Transported 125I-NGF was quantified by collecting all radioactivity from center compartments (medium + cell bodies/proximal axons) after the 15–24 hr incubations with 0.2–40 ng/ml 125I-NGF. Total transport was divided by the transport interval and then compared with the amount of125I-NGF associated with distal axons at steady-state (100%) to determine the transport rate (%/hr). Concentrations of applied 125I-NGF are 0.2 ng/ml (circles), 3–20 ng/ml (squares), and 40 ng/ml (triangles). Error bars ± SEM fall within symbols when not visible. A linear regression was calculated from data from a total of 17 experiments.
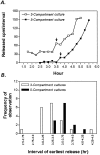
Fig. 5.
Velocity of retrograde transport. In both three- and five-compartment cultures (shown in Fig. 1_a,b_), distal axons were incubated with 10–65 ng/ml 125I-NGF. At 15 min intervals, medium from the cell body/proximal axon compartment was exchanged, and the radioactivity in the medium aliquots was quantified. A, Shown are representative cultures in which the first appearance of radioactivity above background (representing release by the neurons) is marked by an_arrow_. B, Cumulative data show when radioactivity was first released from cell bodies/proximal axons. The 15–30 min delay in five-compartment cultures was attributed to transport across the intermediate axon compartment spanning 5 mm. Transport velocity was estimated as 5 mm/(0.25–0.5 hr) = 10–20 mm/hr.
Fig. 6.
Lack of release of NGF or its degradation products by intermediate axons. In five-compartment cultures (shown in Fig.1_c_), distal axons were incubated with 10–50 ng/ml125I-NGF for 22–40 hr, during which 125I-NGF was retrogradely transported through intermediate axons and into cell bodies/proximal axons. Then radioactivity was collected from various fractions, as shown. Data are expressed as percentage of total combined radioactivity from both compartments. Shown are means ± SEM of 13 cultures.
Fig. 7.
Time course of NGF degradation by pulse–chase analysis. A, Distal axons of 26-d-old three-compartment cultures were pulsed with 200 ng/ml 125I-NGF for 5 hr, during which 125I-NGF associated with distal axons and retrogradely accumulated in cell bodies/proximal axons. After the pulse, distal axons were left intact in some cultures (circles) or were removed by axotomy in other cultures (triangles), and the 125I-NGF-containing medium was replaced with medium containing 200 ng/ml NGF. Radioactivity in the medium bathing cell bodies/proximal axons, representing125I-NGF degradation products, was quantified repetitively in the same cultures by medium exchange at the times shown. Data are expressed as percentages of the total cumulative release. Mean values from one of three experiments (±SEM; 3 cultures/group) are shown.B, Kinetic analysis of the estimated decay of125I-NGF from cell bodies/proximal axons from cultures in which distal axons were absent during the chase (see Materials and Methods). Results from three separate experiments, indicated by different symbols, are shown (9 cultures total).
Similar articles
- Binding, internalization, and retrograde transport of 125I-nerve growth factor in cultured rat sympathetic neurons.
Claude P, Hawrot E, Dunis DA, Campenot RB. Claude P, et al. J Neurosci. 1982 Apr;2(4):431-42. doi: 10.1523/JNEUROSCI.02-04-00431.1982. J Neurosci. 1982. PMID: 7069465 Free PMC article. - Rapid retrograde tyrosine phosphorylation of trkA and other proteins in rat sympathetic neurons in compartmented cultures.
Senger DL, Campenot RB. Senger DL, et al. J Cell Biol. 1997 Jul 28;138(2):411-21. doi: 10.1083/jcb.138.2.411. J Cell Biol. 1997. PMID: 9230082 Free PMC article. - NGF uptake and retrograde signaling mechanisms in sympathetic neurons in compartmented cultures.
Campenot RB. Campenot RB. Results Probl Cell Differ. 2009;48:141-58. doi: 10.1007/400_2009_7. Results Probl Cell Differ. 2009. PMID: 19343309 Review. - Retrograde transport of neurotrophins: fact and function.
Campenot RB, MacInnis BL. Campenot RB, et al. J Neurobiol. 2004 Feb 5;58(2):217-29. doi: 10.1002/neu.10322. J Neurobiol. 2004. PMID: 14704954 Review.
Cited by
- Production of compartmented cultures of rat sympathetic neurons.
Campenot RB, Lund K, Mok SA. Campenot RB, et al. Nat Protoc. 2009;4(12):1869-87. doi: 10.1038/nprot.2009.210. Nat Protoc. 2009. PMID: 20010935 - Sorting of internalized neurotrophins into an endocytic transcytosis pathway via the Golgi system: Ultrastructural analysis in retinal ganglion cells.
Butowt R, von Bartheld CS. Butowt R, et al. J Neurosci. 2001 Nov 15;21(22):8915-30. doi: 10.1523/JNEUROSCI.21-22-08915.2001. J Neurosci. 2001. PMID: 11698603 Free PMC article. - The structural effect of systemic NGF treatment on permanently axotomised dorsal root ganglion cells in adult rats.
Tandrup T, Vestergaard S, Tomlinson DR, Diemel LT, Jakobsen J. Tandrup T, et al. J Anat. 1999 Apr;194 ( Pt 3)(Pt 3):373-9. doi: 10.1046/j.1469-7580.1999.19430373.x. J Anat. 1999. PMID: 10386775 Free PMC article. - NGF Signaling in Endosomes.
Yamashita N. Yamashita N. Adv Exp Med Biol. 2021;1331:19-29. doi: 10.1007/978-3-030-74046-7_3. Adv Exp Med Biol. 2021. PMID: 34453290 - Regulation of trafficking of activated TrkA is critical for NGF-mediated functions.
Yu T, Calvo L, Anta B, López-Benito S, Southon E, Chao MV, Tessarollo L, Arévalo JC. Yu T, et al. Traffic. 2011 Apr;12(4):521-34. doi: 10.1111/j.1600-0854.2010.01156.x. Epub 2011 Feb 1. Traffic. 2011. PMID: 21199218 Free PMC article.
References
- Abbate SL, Atkinson MB, Breuer AC. Amount and speed of fast axonal transport in diabetes. Diabetes. 1991;40:111–117. - PubMed
- Auletta M, Nielsen FC, Gammeltoft S. Receptor-mediated endocytosis and degradation of insulin-like growth factor I and II in neonatal rat astrocytes. J Neurosci Res. 1992;31:14–20. - PubMed
- Bernd P, Greene LA. Association of 125I-nerve growth factor with PC12 pheochromocytoma cells. J Biol Chem. 1984;259:15509–15516. - PubMed
- Breuer AC, Lynn MP, Atkinson MB, Chou SM, Wilbourn AJ, Marks KE, Culver JE, Fleegler EJ. Fast axonal transport in amyotrophic lateral sclerosis: an intra-axonal organelle traffic analysis. Neurology. 1987;37:738–747. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources