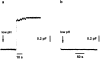An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids - PubMed (original) (raw)
An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids
L V Chernomordik et al. J Cell Biol. 1997.
Abstract
While the specificity and timing of membrane fusion in diverse physiological reactions, including virus-cell fusion, is determined by proteins, fusion always involves the merger of membrane lipid bilayers. We have isolated a lipid-dependent stage of cell-cell fusion mediated by influenza hemagglutinin and triggered by cell exposure to mildly acidic pH. This stage preceded actual membrane merger and fusion pore formation but was subsequent to a low pH-induced change in hemagglutinin conformation that is required for fusion. A low pH conformation of hemagglutinin was required to achieve this lipid-dependent stage and also, downstream of it, to drive fusion to completion. The lower the pH of the medium applied to trigger fusion and, thus, the more hemagglutinin molecules activated, the less profound was the dependence of fusion on lipids. Membrane-incorporated lipids affected fusion in a manner that correlated with their dynamic molecular shape, a characteristic that determines a lipid monolayer's propensity to bend in different directions. The lipid sensitivity of this stage, i.e., inhibition of fusion by inverted cone-shaped lysophosphatidylcholine and promotion by cone-shaped oleic acid, was consistent with the stalk hypothesis of fusion, suggesting that fusion proteins begin membrane merger by promoting the formation of a bent, lipid-involving, stalk intermediate.
Figures
Figure 1
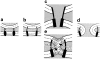
Two hypothetical pathways in HA-mediated fusion. (a) HA-expressing membrane in contact with the target membrane. (b) Low pH induces dramatic changes in HA conformation including insertion of the HA fusion peptide into the target membrane. (c) Formation of a proteinaceous pore. (d) Completion of the fusion process. Dashed lines show the boundaries of the hydrophobic surfaces of monolayers. (e) Local merger of membrane outer monolayers. According to the stalk hypothesis, a transient and local connection between membranes (stalk) has a net negative curvature and its formation should be facilitated and hindered by cone-shaped lipids (e.g., OA), and by inverted cone–shaped lipids (e.g., LPC), respectively. Dashed lines show the boundaries of the hydrophobic surfaces of two monolayers.
Figure 6
The inhibition of pore formation by LPC. Fusion of a HAb2 cell with a single RBC. The arrows indicate the beginning of the application of pH 4.9 solution. Note that these experiments were performed at 33°C rather than at room temperature to accelerate fusion pore formation upon low pH application (Spruce et al., 1989). (a) No LPC added. The increase in capacitance reflects the fusion event. The waiting time between the beginning of low pH application and the onset of the capacitance increase was 16.8 ± 8.9 s (n = 10). (b) Capacitance trace in the presence of 25 μM lauroyl LPC.
Figure 8
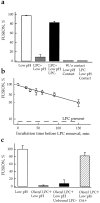
Lipids affect fusion downstream of the triggering step. (a) LPC reversibly arrested fusion downstream low pH–dependent stages. Fusion was triggered by a 2-min application of pH 4.9 medium and assayed by fluorescence microscopy as R18 redistribution. □, fusion of HAb2 cells with prebound RBCs, no lipids added; ▒⃞ , starting 5 min before the low pH pulse, cells were incubated in the presence of 150 μM lauroyl LPC; ▪, LPC was washed out by LPC-free PBS 20 min after the end of low pH application; ▨ , the same pulse of low pH was applied to a suspension of HAb2 cells in the absence of RBCs. Then, back at neutral pH , R18-labeled RBCs were added to the low pH–treated HAb2 cells. ▨ , similar to the previous experiment, but low pH was applied to HAb2 cells in the presence of 150 μM lauroyl LPC, then R18-labeled RBCs were added at neutral pH but still in the presence of LPC, and, finally, HAb2 cells with bound RBCs were washed out with LPC-free PBS to remove lysolipid. Fusion was assayed 20 min later. (b) Slow inactivation of the committed fusion stage arrested by LPC. Fusion of HAb2 cells with prebound PKH26-labeled RBCs was triggered in the presence of 150 μM lauroyl LPC by a 2-min application of pH 4.9 medium. At different time intervals after low pH application, LPC was removed by washing cells with LPC-free PBS. t = 0 corresponds to the end of the low pH pulse. The extent of fusion observed at t = 2 h in the experiment when LPC was not removed is shown by dashed line. Fusion extents assayed by fluorescence microscopy as PKH26 redistribution were normalized to those in control experiments with no lipids added. Each point is mean ± SEM, n = 3. (c) Adding OA released cells from the LPC-arrested fusion stage. Cells were triggered to fuse by a 2-min treatment with pH 4.95 medium in the absence or in the presence of 9.6 μM oleoyl LPC and then returned to the LPC-free neutral pH medium. The recovery of fusion previously arrested by LPC reached an extent close to that of the control experiment (no lipids added) upon addition of 5 μM OA at neutral pH , 5 min after the end of low pH pulse. No fusion was observed in control experiments when OA was added to cells not treated by low pH . Thus, OA canceled the LPC block to low pH–triggered fusion rather than having induced fusion on its own. Each point is mean ± SEM, n = 3.
Figure 2
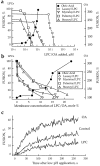
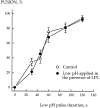
Inhibition and promotion of fusion by lipids. (a and b) The extent of R18-labeled RBC fusion to HAb2 cells in the presence of OA or LPCs with different hydrocarbon chains. In this representative experiment, fusion was triggered by lowering the pH to 4.9. Fusion, assayed by spectrofluorometry, was plotted vs. concentration of exogenous lipids in the medium (a) or in RBC membranes (b). Membrane concentration of exogenous lipids was estimated from R18 dequenching as described in Materials and Methods. (c) Effects of oleoyl LPC (2.5 μM) and OA (10 μM) on the kinetics of membrane mixing. The increase of fluorescence with time after lowering the pH to 5.0 reflects dilution of self-quenched R18 upon lipid mixing between labeled RBCs and unlabeled HAb2 cells upon their fusion.
Figure 3
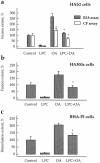
LPC and OA added together compensate the effects of each other on lipid mixing and content mixing mediated by different forms of HA. Fusion of HA-expressing cells (HAb2 [_a_] and HA300a [_b_]) and hemifusion of GPI-HA–expressing cells (BHA-PI [_c_]) with prebound RBCs labeled by either membrane dye R18 or aqueous content marker CF was studied with no added lipids (Control) or in the presence of oleoyl LPC (50 μM), OA (50 μM), or both LPC and OA (same concentrations). Fusion was triggered by a 2-min application of low pH medium (pH 5.2 for HAb2 and HA300a cells, and pH 5.25 for BHA-PI cells). Fusion extents, assayed by fluorescence microscopy as R18 redistribution (hatched bars) or as CF redistribution (open bars), were normalized to those in control experiments: 36.1, 26.8, 13.2, and 17.7% for HAb2, HA300a and BHA-PI (all -R18 assay), and HAb2- CF assay, respectively. Each bar is mean ± SEM, n = 3. Bars which were not statistically different from the corresponding controls are labeled by asterisks. In a, fusion extent for HAb2 (CF assay) in the presence of OA alone was significantly higher than that in the presence of both OA and LPC (P < 0.05).
Figure 4
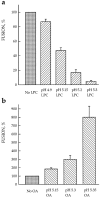
The lower the pH applied to trigger fusion, the less profound is the dependence of fusion on lipids. Fusion of HAb2 cells with prebound RBCs was triggered by a 2-min application of low pH medium in the presence of 19 μM stearoyl LPC (a) or 25 μM OA (b). The extent of fusion assayed by fluorescence microscopy as R18 redistribution was normalized to those in the corresponding control experiments with no lipids added: (a) 98 ± 0.5%, 68 ± 3%, 53 ± 4%, and 36 ± 4.5% for pH 4.9, 5.15, 5.2, and 5.3, respectively; (b) 39 ± 3%, 15 ± 3%, and 3 ± 0.5% for pH 5.15, 5.3, and 5.35, respectively. Each point is mean ± SEM, n = 3. Note that because of day-to-day variability (see Materials and Methods), fusion efficiency observed for the same suboptimal pH values with no exogenous lipids added was somehow different in two independent experiments presented in a and b.
Figure 5
LPC incorporated into either one or both of two fusing membranes reversibly inhibits fusion. Fusion was triggered by a 10-min application of pH 4.9 medium and assayed by fluorescence microscopy as R18 redistribution. Each bar is mean ± SEM, n = 3. (a) Fusion inhibition is caused by membrane-incorporated rather than unbound LPC. □, fusion of HAb2 cells with prebound RBCs, no lipids added; ▒⃞ , fusion in the presence of 36 μM stearoyl LPC; ▪, unbound LPC was removed before low pH application. Cells were incubated with LPC (same concentration as above) for 10 min and then washed out with LPC-free PBS; ▨ , LPC was extracted from membranes by BSA before low pH application. Cells were incubated with LPC as above and then washed out with LPC-free PBS containing BSA. Each point is mean ± SEM, n = 3. (b) LPC inhibits fusion when present either in HA-expressing membrane or in RBC membrane. □, fusion of HAb2 cells with RBCs, no lipids added, ▒⃞ , only HAb2 cells were treated by stearoyl LPC (42 μM); ▪, only RBCs were treated by stearoyl LPC (1 μM/5 × 106 RBCs); ▨ , both RBCs and HAb2 cells were separately treated with stearoyl LPC (same concentrations as above).
Figure 7
LPC does not affect the low pH–dependent conformational changes of HA required for fusion. HAb2 cells with prebound R18-labeled RBCs were incubated in low pH medium (pH 5.2), LPC-free, or supplemented with 150 μM lauroyl LPC for different times. At the end of the low pH pulse, the low pH medium was replaced by LPC-free PBS (pH 7.4) to both terminate the fusion-triggering pulse and to wash out LPC. Fusion was assayed by fluorescence microscopy as R18 redistribution. Each point is mean ± SEM, n = 3. In the experiments when the PBS used to terminate the low pH pulse was supplemented with 150 μM lauroyl LPC, and thus LPC was not washed out, the extent of fusion was 0.2 ± 0.16% (n = 3).
Figure 9
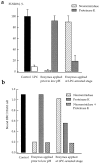
Effects of neuraminidase and proteinase K on the LPCarrested stage of HA-mediated fusion. Fusion of HAb2 cells with prebound R18-labeled RBCs was triggered by a 3-min pH 4.9 pulse applied in the presence of 50 μM lauroyl LPC. After a 20min incubation of cells at neutral pH in LPC-containing PBS, lysolipid was removed by washing cells with LPC-free PBS. Cells were treated by neuraminidase or proteinase K before low pH application or at the LPC-arrested fusion stage in the time interval between the end of low pH and removal of LPC. (a) Fusion. The extent of fusion after removal of LPC was measured as R18 redistribution using fluorescence microscopy, and normalized to those in the control experiments (▪, neither LPC nor enzymes applied). □, the extent of fusion observed at t = 2 hours in the experiments when LPC was not removed. ▨ and ▒⃞ , cells were treated with neuraminidase or proteinase K, respectively. Each point is mean ± SEM, n = 3. (b) Binding. In this representative experiment, the effects of neuraminidase (▨ ) or proteinase K (▒⃞ ), or the combination of both enzymes (▩ ) on binding of R18labeled RBCs to HAb2 cells before low pH application and during the LPC-arrested fusion stage were evaluated with fluorescence microscopy by counting RBCs bound to ∼100 HAb2 cells. ▪, An average number of RBCs bound per one HAb2 cell in the control experiments with neither LPC nor enzymes applied.
Similar articles
- Liposome composition effects on lipid mixing between cells expressing influenza virus hemagglutinin and bound liposomes.
Bailey A, Zhukovsky M, Gliozzi A, Chernomordik LV. Bailey A, et al. Arch Biochem Biophys. 2005 Jul 15;439(2):211-21. doi: 10.1016/j.abb.2005.05.010. Arch Biochem Biophys. 2005. PMID: 15963452 - The 1-127 HA2 construct of influenza virus hemagglutinin induces cell-cell hemifusion.
Leikina E, LeDuc DL, Macosko JC, Epand R, Epand R, Shin YK, Chernomordik LV. Leikina E, et al. Biochemistry. 2001 Jul 27;40(28):8378-86. doi: 10.1021/bi010466+. Biochemistry. 2001. PMID: 11444985 - Non-bilayer lipids and biological fusion intermediates.
Chernomordik L. Chernomordik L. Chem Phys Lipids. 1996 Jul 15;81(2):203-13. doi: 10.1016/0009-3084(96)02583-2. Chem Phys Lipids. 1996. PMID: 8810049 Review. - Short-chain alcohols promote an early stage of membrane hemifusion.
Chanturiya A, Leikina E, Zimmerberg J, Chernomordik LV. Chanturiya A, et al. Biophys J. 1999 Oct;77(4):2035-45. doi: 10.1016/S0006-3495(99)77044-X. Biophys J. 1999. PMID: 10512823 Free PMC article. - Structural intermediates in influenza haemagglutinin-mediated fusion.
Chernomordik LV, Leikina E, Kozlov MM, Frolov VA, Zimmerberg J. Chernomordik LV, et al. Mol Membr Biol. 1999 Jan-Mar;16(1):33-42. doi: 10.1080/096876899294733. Mol Membr Biol. 1999. PMID: 10332735 Review.
Cited by
- Novel insight into the lipid network of plasma extracellular vesicles reveal sex-based differences in the lipidomic profile of alcohol use disorder patients.
Perpiñá-Clérigues C, Mellado S, Galiana-Roselló C, Fernández-Regueras M, Marcos M, García-García F, Pascual M. Perpiñá-Clérigues C, et al. Biol Sex Differ. 2024 Jan 25;15(1):10. doi: 10.1186/s13293-024-00584-5. Biol Sex Differ. 2024. PMID: 38273378 Free PMC article. - Fast lipid disorientation at the onset of membrane fusion revealed by molecular dynamics simulations.
Ohta-Iino S, Pasenkiewicz-Gierula M, Takaoka Y, Miyagawa H, Kitamura K, Kusumi A. Ohta-Iino S, et al. Biophys J. 2001 Jul;81(1):217-24. doi: 10.1016/S0006-3495(01)75693-7. Biophys J. 2001. PMID: 11423408 Free PMC article. - Electrospray ionization mass spectrometry analysis of changes in phospholipids in RBL-2H3 mastocytoma cells during degranulation.
Ivanova PT, Cerda BA, Horn DM, Cohen JS, McLafferty FW, Brown HA. Ivanova PT, et al. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7152-7. doi: 10.1073/pnas.131195098. Proc Natl Acad Sci U S A. 2001. PMID: 11416200 Free PMC article. - Cholesterol promotes hemifusion and pore widening in membrane fusion induced by influenza hemagglutinin.
Biswas S, Yin SR, Blank PS, Zimmerberg J. Biswas S, et al. J Gen Physiol. 2008 May;131(5):503-13. doi: 10.1085/jgp.200709932. J Gen Physiol. 2008. PMID: 18443361 Free PMC article. - A mechanism of protein-mediated fusion: coupling between refolding of the influenza hemagglutinin and lipid rearrangements.
Kozlov MM, Chernomordik LV. Kozlov MM, et al. Biophys J. 1998 Sep;75(3):1384-96. doi: 10.1016/S0006-3495(98)74056-1. Biophys J. 1998. PMID: 9726939 Free PMC article.
References
- Alford D, Ellens H, Bentz J. Fusion of influenza virus with sialic acid-bearing target membranes. Biochemistry. 1994;33:1977–1987. - PubMed
- Bhamidipati SP, Hamilton JA. Interactions of lyso 1-palmitoylphosphatidylcholine with phospholipids: a 13C and 31P NMR study. Biochemistry. 1995;34:5666–5677. - PubMed
- Broring K, Haest CW, Deuticke B. Translocation of oleic acid across the erythrocyte membrane. Evidence for a fast process. Biochim Biophys Acta. 1989;986:321–331. - PubMed
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature (Lond) 1994;371:37–43. - PubMed
- Carr CM, Kim PS. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. - PubMed