Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle - PubMed (original) (raw)
Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle
C Thiemermann et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Reperfusion of the ischemic myocardium results in the generation of oxygen-derived free radicals, NO, and presumably peroxynitrite. These, in turn, may cause strand breaks in DNA, which activate the nuclear enzyme poly(ADP ribose) synthetase (PARS). This results in a rapid depletion of intracellular NAD and ATP. When this reaction is excessive, there is ultimately cell death. Here we demonstrate that 3-aminobenzamide (and several other, chemically distinct, inhibitors of PARS activity) reduces the infarct size caused by ischemia and reperfusion of the heart or skeletal muscle of the rabbit. Inhibition of PARS activity also attenuates the myocardial dysfunction caused by global ischemia and reperfusion in the isolated, perfused heart of the rabbit. In skeletal muscle, inhibition of the activity of neuronal NO synthase reduces infarct size, indicating that the formation of NO contributes to the activation of PARS there. There is no significant neuronal NO synthase activity in the heart, and hence NO synthase inhibitors did not reduce myocardial infarct size. Thus, activation of PARS contributes to the cell death caused by ischemia-reperfusion, and PARS inhibitors may constitute a novel therapy for ischemia-reperfusion injury.
Figures
Figure 1
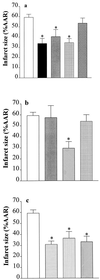
Inhibitors of PARS activity reduce myocardial infarct size in the rabbit. (a) When compared with controls (open column; n = 9), 3-AB (10 mg·kg−1 i.a.) reduced infarct size when given (i) 1 min before LAL occlusion and 1 min before reperfusion (black column, n = 9); (ii) 1 min before occlusion (gray column, n = 6); or (iii) 1 min before reperfusion (grid-patterned column, n = 7). In contrast, 3-aminobenzoic acid (10 mg·kg−1 i.a; given 1 min before reperfusion; cross-hatched column, n = 5) did not reduce infarct size. (b) When compared with controls (open column; n = 9), i.a. injection 1 min before reperfusion of nicotinamide (20 mg·kg−1 i.a., hatched column, n = 5), but not of 10 mg·kg−1 (light gray column, n = 3) caused a reduction in infarct size. Furthermore, nicotinic acid (20 mg·kg−1 i.a., grid-patterned column, n = 5) had no effect on infarct size. (c) The PARS inhibitors 4-aminobenzamide (10 mg·kg−1, horizontal striped column, n = 5), 4-amino-1,8-naphthalimide (1 mg·kg−1, vertical striped column, n = 5), and 1,5-dihydroxyisoquinoline (1 mg·kg−1, hatched column, n = 5) also caused reductions in infarct size. ∗, P < 0.05 compared with control (ANOVA followed by Bonferoni’s test).
Figure 2
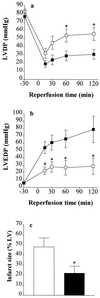
The PARS inhibitor 3-AB attenuates the contractile dysfunction and reduces infarct size of hearts subjected to global ischemia and reperfusion. Thirty minutes of global ischemia followed by 2 h of reperfusion (▪, vehicle control, n = 7) resulted in (a) a substantial impairment of left ventricular developed pressure (LVDP) and (b) a rise in left ventricular end diastolic pressure (LVEDP) in the isolated, perfused heart of the rabbit. Infusion (into the perfusion buffer) of 3-AB (□, final concentration 100 μM, n = 6) resulted in a significant improvement in the recovery of contractile function and attenuated the rise in LVEDP. ∗, P < 0.05, ANOVA followed by Bonferoni’s test when compared with vehicle control. (c) When compared with control (open column, n = 7), 3-AB (solid column, n = 6) caused a significant reduction in infarct size.
Figure 3
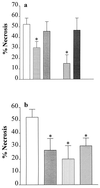
Inhibitors of PARS activity reduce the degree of skeletal muscle (gracilis) necrosis in a rabbit model of hind limb ischemia (4 h) and reperfusion (3 h). (a) When compared with vehicle (saline)-treated controls (open column; n = 18), the PARS inhibitor 3-AB (10 mg·kg−1 i.v., grid-patterned column, n = 16), but not its inactive analogue 3-aminobenzoic acid (10 mg·kg−1 i.v., gray column, n = 8), reduces skeletal muscle necrosis. Similarly, nicotinamide (20 mg·kg−1 i.v., hatched column, n = 6), but not nicotinic acid (20 mg·kg−1 i.v., dark gray column, n = 6), reduces skeletal muscle necrosis. (b) The PARS inhibitors benzamide (1 mg·kg−1 i.v., gray column, n = 10), 4-amino-1,8-naphthalimide (1 mg·kg−1 i.v., vertical striped column, n = 6), and 1,5-dihydroxyisoquinoline (1 mg·kg−1 i.v., hatched column, n = 10) also cause a significant reduction in the degree of skeletal muscle necrosis. ∗, P < 0.05 when compared with vehicle control by ANOVA followed by Bonferoni’s test.
Figure 4
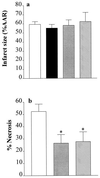
Inhibition of NOS activity reduces infarct size in skeletal muscle, but not in the heart. (a) When compared with vehicle-treated controls (open column, n = 9), the NOS inhibitors _N_G-methyl-
l
-arginine (3 mg·kg−1 i.a., black column, n = 3), 7-NI (30 mg·kg−1 i.p., gray column, n = 5), and AE-ITU (10 mg·kg−1 i.a., hatched column, n = 3) did not reduce infarct size in the heart. (b) When compared with vehicle-treated controls (open column; n = 18), the NOS inhibitors 7-NI (30 mg·kg−1 i.p., gray column, n = 10) and AE-ITU (10 mg·kg−1 i.v., hatched column, n = 12) caused a significant reduction in the degree of skeletal muscle infarction. ∗, P < 0.05 compared with control (ANOVA followed by Bonferoni’s test).
Similar articles
- Myocardial ischemic preconditioning in rodents is dependent on poly (ADP-ribose) synthetase.
Liaudet L, Yang Z, Al-Affar EB, Szabó C. Liaudet L, et al. Mol Med. 2001 Jun;7(6):406-17. Mol Med. 2001. PMID: 11474134 Free PMC article. - Inhibitors of poly (ADP-ribose) synthetase protect rat cardiomyocytes against oxidant stress.
Bowes J, McDonald MC, Piper J, Thiemermann C. Bowes J, et al. Cardiovasc Res. 1999 Jan;41(1):126-34. doi: 10.1016/s0008-6363(98)00221-1. Cardiovasc Res. 1999. PMID: 10325960 - Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion.
Szabó C, Dawson VL. Szabó C, et al. Trends Pharmacol Sci. 1998 Jul;19(7):287-98. doi: 10.1016/s0165-6147(98)01193-6. Trends Pharmacol Sci. 1998. PMID: 9703762 Review. - Role of poly(ADP-ribose)synthetase in inflammation.
Szabó C. Szabó C. Eur J Pharmacol. 1998 May 29;350(1):1-19. doi: 10.1016/s0014-2999(98)00249-0. Eur J Pharmacol. 1998. PMID: 9683009 Review.
Cited by
- Age-related endothelial dysfunction : potential implications for pharmacotherapy.
Matz RL, Andriantsitohaina R. Matz RL, et al. Drugs Aging. 2003;20(7):527-50. doi: 10.2165/00002512-200320070-00005. Drugs Aging. 2003. PMID: 12749750 Review. - Myocardial protection by selective poly(ADP-ribose) polymerase inhibitors.
Kovacs K, Toth A, Deres P, Kalai T, Hideg K, Sumegi B. Kovacs K, et al. Exp Clin Cardiol. 2004 Spring;9(1):17-20. Exp Clin Cardiol. 2004. PMID: 19641691 Free PMC article. - Failure of poly(ADP-ribose) polymerase cleavage by caspases leads to induction of necrosis and enhanced apoptosis.
Herceg Z, Wang ZQ. Herceg Z, et al. Mol Cell Biol. 1999 Jul;19(7):5124-33. doi: 10.1128/MCB.19.7.5124. Mol Cell Biol. 1999. PMID: 10373561 Free PMC article. - Suppression of poly (ADP-ribose) polymerase activation by 3-aminobenzamide in a rat model of myocardial infarction: long-term morphological and functional consequences.
Liaudet L, Szabó E, Timashpolsky L, Virág L, Cziráki A, Szabó C. Liaudet L, et al. Br J Pharmacol. 2001 Aug;133(8):1424-30. doi: 10.1038/sj.bjp.0704185. Br J Pharmacol. 2001. PMID: 11498530 Free PMC article. - Effects of inhibitors of the activity of poly (ADP-ribose) synthetase on the organ injury and dysfunction caused by haemorrhagic shock.
McDonald MC, Filipe HM, Thiemermann C. McDonald MC, et al. Br J Pharmacol. 1999 Nov;128(6):1339-45. doi: 10.1038/sj.bjp.0702928. Br J Pharmacol. 1999. PMID: 10578150 Free PMC article.
References
- Satoh M S, Lindahl T. Nature (London) 1992;356:356–358. - PubMed
- Ikai K, Ueda K. J Histochem Cytochem. 1983;31:1261–1264. - PubMed
- Hyslop P A, Hinshaw D B, Halsey W A, Schraufstatter I U, Sauerheber R D, Spraggs R G, Jackson J H, Cochrane C G. J Biol Chem. 1988;263:1665–1675. - PubMed
- Thies R L, Autor A P. Arch Biochem Biophys. 1991;286:353–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous