The vertebrate GLFG nucleoporin, Nup98, is an essential component of multiple RNA export pathways - PubMed (original) (raw)
The vertebrate GLFG nucleoporin, Nup98, is an essential component of multiple RNA export pathways
M A Powers et al. J Cell Biol. 1997.
Abstract
The 97-kD O-linked glycoprotein, Nup98, is a component of the Xenopus laevis nuclear pore complex and the only vertebrate GLFG nucleoporin identified (Powers, M.A., C. Macauley, F. Masiarz, and D.J. Forbes. 1995. J. Cell Biol. 128:721-736). We have investigated possible roles of xNup98 in the nucleocytoplasmic transport of proteins and RNAs by analyzing the consequences of injecting monospecific polyclonal antibodies to xNup98 into X. laevis oocytes. We show here that nuclear injection of anti-xNup98 inhibited the export of multiple classes of RNAs, including snRNAs, 5S RNA, large ribosomal RNAs, and mRNA. In contrast, the export of tRNA was unaffected. Injection of anti-xNup98 into the oocyte cytoplasm had no effect on export of any of the RNAs. Significantly, nuclear injection of anti-xNup98 antibodies did not inhibit import of either karyophilic proteins or snRNPs. This latter result is in agreement with our previous finding that Nup98 is not an essential element of the protein import pathway. Thus, Nup98 plays a role specifically in RNA export from the nucleus, and it appears to be an essential component of multiple RNA export pathways.
Figures
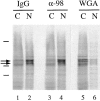
Figure 1
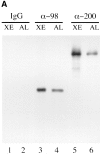
Specificity of affinity-purified anti-xNup98 antibodies. (A) Affinity purified anti-nucleoporin antibodies (α-98 and α-200) or a nonspecific rabbit IgG was used to probe immunoblots containing Xenopus WGA binding proteins (Xenopus eluate, XE) and a nuclear pore fraction from Xenopus annulate lamellae (AL). (B) Affinity purified anti-xNup98 was used to probe blots containing either E. coli lysate (control), lysate from strains expressing regions of rat Nup98, or Xenopus cytosol (XC). rNup98 corresponds to the nearly full length protein, amino acids 43–824. The GLFG repeat domain (GLFG) corresponds to amino acids 43–490. The RNA binding domain (RBD) corresponds to amino acids 618–824.
Figure 1
Specificity of affinity-purified anti-xNup98 antibodies. (A) Affinity purified anti-nucleoporin antibodies (α-98 and α-200) or a nonspecific rabbit IgG was used to probe immunoblots containing Xenopus WGA binding proteins (Xenopus eluate, XE) and a nuclear pore fraction from Xenopus annulate lamellae (AL). (B) Affinity purified anti-xNup98 was used to probe blots containing either E. coli lysate (control), lysate from strains expressing regions of rat Nup98, or Xenopus cytosol (XC). rNup98 corresponds to the nearly full length protein, amino acids 43–824. The GLFG repeat domain (GLFG) corresponds to amino acids 43–490. The RNA binding domain (RBD) corresponds to amino acids 618–824.
Figure 2
Differential effects of anti-xNup98 antibodies on transport of in vivo synthesized RNA polymerase II and III transcripts. A mixture of DNA templates encoding U4 snRNA, 5S rRNA, and tRNATyr was injected into oocyte nuclei together with buffer (Control) or monospecific antibodies against nucleoporins xNup98 (α-98) or xNup200 (α200). 2 h later, α[32P]GTP (∼1.0 μCi/oocyte) was injected into the cytoplasms of all oocytes. After 4 (A) or 21 h of labeling (B), oocytes (3–6/timepoint) were manually dissected, and the intracellular distributions of the newly made RNAs were determined by analyses of 0.5 oocyte equivalents of the nuclear (N) and cytoplasmic (C) RNAs in 8% denaturing polyacrylamide gels. The RNAs made from the injected genes are indicated. Endogenous oocyte RNAs made in the absence of injected genes (No DNA) are shown in B, lanes 7 and 8. The level of 5S DNA templates was ∼10-fold higher in A than in B.
Figure 4
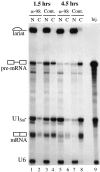
Inhibition of mRNA export by anti-xNup98 antibodies. Anti-xNup98 antibodies (α-98) or buffer (Cont.) was injected into oocyte nuclei and 2 h later the same oocytes received a second nuclear injection with a mixture of 32P-labeled AdML premRNA, U1Sm− RNA, and U6 RNA that had been synthesized in vitro using SP6 or T7 RNA polymerases. At 1.5 (lanes 1–4) and 4.5 h (lanes 5–8) after RNA injection, splicing of the AdML premRNA and export of the spliced mRNA and U1Sm− RNA were monitored by analyses of nuclear (N) and cytoplasmic (C) RNAs isolated from individual oocytes. The injected mixture of RNAs (Inj.) is shown in lane 9. U1Sm− RNA, which lacks a functional Sm binding site, is exported to the cytoplasm but cannot be imported back into the nucleus. U6 RNA is retained in the nucleus and serves as a marker for the accuracy of the second nuclear injection and oocyte dissections.
Figure 3
Failure of snRNA precursors to leave the nucleus of anti-xNup98 treated oocytes. Total nuclear RNAs (T), corresponding to those in lanes 1 and 3 of Fig. 2, A and B, were immunoprecipitated with anti-m7G antibodies, which are specific for the cap structure of the nonexported, nuclear precursor snRNA. The RNAs in the precipitate (P) and supernatant (S) fractions were analyzed as in Fig. 2. Dots by lane 12 indicate m7G-capped degradation products of pre-U4 RNA that also are observed when nuclear export is inhibited by other reagents.
Figure 5
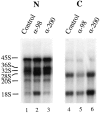
Reduced export of ribosomal RNAs in the presence of anti-xNup98 antibodies. Buffer (Control) or antibodies specific for xNup98 (α-98) or xNup200 (α-200) were injected into oocyte nuclei. 2 h later α[32P]GTP (∼1.0 μCi/oocyte) was injected into the cytoplasm of all oocytes. After 20 h of labeling, the intracellular distribution of newly made endogenous ribosomal RNAs was determined by analyses of 0.5 oocyte equivalents of nuclear (lanes 1–3) and cytoplasmic RNAs (lanes 4–6) in a 1.2% formaldehyde–agarose gel. The electrophoretic mobilities of precursor (45, 36, 32, and 20S) and mature (18 and 28S) ribosomal RNAs are indicated.
Figure 6
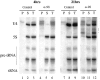
Lack of effect of anti-xNup98 antibodies on import of karyophilic proteins. Normal IgG (IgG), anti-xNup98 (α-98), or WGA was injected into oocyte nuclei (IgG, α-98) or cytoplasms (WGA). 2 h later, 0.25 oocyte equivalents of 35S-labeled total soluble nuclear proteins were injected into the cytoplasm of all oocytes. After 24 h incubation, oocytes were manually dissected, and total nuclear (N) and cytoplasmic (C) extracts were prepared from pools of 3–5 oocytes. 0.5 oocyte equivalents of the extracts were analyzed on a 10% polyacrylamide gel containing SDS and 35S-labeled proteins were detected by autoradiography. Arrows indicate the strongly labeled karyophilic proteins N1/N2 (Dabauvalle and Francke, 1982). Lines indicate the positions of molecular weight markers of 200, 97, and 66 kD.
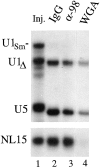
Figure 7
Unimpaired import of snRNPs and small RNAs in the presence of anti-xNup98 antibodies. Oocytes that had been pre-injected with antibodies or WGA, as in Fig. 6, were injected in the cytoplasm with a mixture of 32P-labeled U1Sm−, U1Δ, U5, and NL15 RNAs that were made by in vitro transcription. After 7 h of incubation, import of the RNAs was monitored by polyacrylamide gel analysis of 0.5 oocyte equivalents of nuclear RNAs isolated from pools of 3–5 oocyte nuclei. The mixture of injected RNAs (Inj.) is shown in lane 1; the slightly faster gel mobilities of U1Δ (a mutant U1 RNA lacking the U1A protein binding site) and U5 RNAs in lanes 2–4 are due to 3′ end trimming which occurs upon nuclear import of snRNPs (Neuman de Vegvar and Dahlberg, 1990). U1Sm− RNA lacks the Sm binding site required for import and serves as a control for cytoplasmic contamination of the nuclear samples. Cytoplasmic fractions are not shown, as RNA levels were in excess, and differences among them were not significant.
Similar articles
- The nucleoporin nup153 plays a critical role in multiple types of nuclear export.
Ullman KS, Shah S, Powers MA, Forbes DJ. Ullman KS, et al. Mol Biol Cell. 1999 Mar;10(3):649-64. doi: 10.1091/mbc.10.3.649. Mol Biol Cell. 1999. PMID: 10069809 Free PMC article. - Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export.
Vasu S, Shah S, Orjalo A, Park M, Fischer WH, Forbes DJ. Vasu S, et al. J Cell Biol. 2001 Oct 29;155(3):339-54. doi: 10.1083/jcb.200108007. Epub 2001 Oct 29. J Cell Biol. 2001. PMID: 11684705 Free PMC article. - Visualizing nuclear export of different classes of RNA by electron microscopy.
Panté N, Jarmolowski A, Izaurralde E, Sauder U, Baschong W, Mattaj IW. Panté N, et al. RNA. 1997 May;3(5):498-513. RNA. 1997. PMID: 9149231 Free PMC article. - Transport of RNA between nucleus and cytoplasm.
Izaurralde E, Mattaj IW. Izaurralde E, et al. Semin Cell Biol. 1992 Aug;3(4):279-88. doi: 10.1016/1043-4682(92)90029-u. Semin Cell Biol. 1992. PMID: 1384772 Review. - Nucleoporin Nup98: a gatekeeper in the eukaryotic kingdoms.
Iwamoto M, Asakawa H, Hiraoka Y, Haraguchi T. Iwamoto M, et al. Genes Cells. 2010 Jun;15(7):661-9. doi: 10.1111/j.1365-2443.2010.01415.x. Epub 2010 Jun 7. Genes Cells. 2010. PMID: 20545767 Review.
Cited by
- NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia.
Lin YW, Slape C, Zhang Z, Aplan PD. Lin YW, et al. Blood. 2005 Jul 1;106(1):287-95. doi: 10.1182/blood-2004-12-4794. Epub 2005 Mar 8. Blood. 2005. PMID: 15755899 Free PMC article. - Distinct functional domains within nucleoporins Nup153 and Nup98 mediate transcription-dependent mobility.
Griffis ER, Craige B, Dimaano C, Ullman KS, Powers MA. Griffis ER, et al. Mol Biol Cell. 2004 Apr;15(4):1991-2002. doi: 10.1091/mbc.e03-10-0743. Epub 2004 Jan 12. Mol Biol Cell. 2004. PMID: 14718558 Free PMC article. - Developmental genetics of the essential Drosophila nucleoporin nup154: allelic differences due to an outward-directed promoter in the P-element 3' end.
Kiger AA, Gigliotti S, Fuller MT. Kiger AA, et al. Genetics. 1999 Oct;153(2):799-812. doi: 10.1093/genetics/153.2.799. Genetics. 1999. PMID: 10511559 Free PMC article. - Nup116p and nup100p are interchangeable through a conserved motif which constitutes a docking site for the mRNA transport factor gle2p.
Bailer SM, Siniossoglou S, Podtelejnikov A, Hellwig A, Mann M, Hurt E. Bailer SM, et al. EMBO J. 1998 Feb 16;17(4):1107-19. doi: 10.1093/emboj/17.4.1107. EMBO J. 1998. PMID: 9463388 Free PMC article. - Crystal structure of the N-terminal domain of Nup358/RanBP2.
Kassube SA, Stuwe T, Lin DH, Antonuk CD, Napetschnig J, Blobel G, Hoelz A. Kassube SA, et al. J Mol Biol. 2012 Nov 9;423(5):752-65. doi: 10.1016/j.jmb.2012.08.026. Epub 2012 Sep 7. J Mol Biol. 2012. PMID: 22959972 Free PMC article.
References
- Adolph KW, Cheng SM, Laemmli UK. Role of nonhistone proteins in metaphase chomosome structure. Cell. 1977;12:805–816. - PubMed
- Cheng Y, Dahlberg JE, Lund E. Diverse effects of the guanine nucleotide exchange factor RCC1 on RNA transport. Science (Wash DC) 1995;267:1807–1810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources