The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase - PubMed (original) (raw)
The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase
B He et al. Proc Natl Acad Sci U S A. 1997.
Abstract
In human cells infected with herpes simplex virus 1 the double-stranded RNA-dependent protein kinase (PKR) is activated but phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2 (eIF-2) and total shutoff of protein synthesis is observed only in cells infected with gamma(1)z34.5- mutants. The carboxyl-terminal 64 aa of gamma(1)34.5 protein are homologous to the corresponding domain of MyD116, the murine growth arrest and DNA damage gene 34 (GADD34) protein and the two domains are functionally interchangeable in infected cells. This report shows that (i) the carboxyl terminus of MyD116 interacts with protein phosphatase 1alpha in yeast, and both MyD116 and gamma(1)34.5 interact with protein phosphatase 1alpha in vitro; (ii) protein synthesis in infected cells is strongly inhibited by okadaic acid, a phosphatase 1 inhibitor; and (iii) the alpha subunit in purified eIF-2 phosphorylated in vitro is specifically dephosphorylated by S10 fractions of wild-type infected cells at a rate 3000 times that of mock-infected cells, whereas the eIF-2alpha-P phosphatase activity of gamma(1)34.5- virus infected cells is lower than that of mock-infected cells. The eIF-2alpha-P phosphatase activities are sensitive to inhibitor 2. In contrast to eIF-2alpha-P phosphatase activity, extracts of mock-infected cells exhibit a 2-fold higher phosphatase activity on [32P]phosphorylase than extracts of infected cells. These results indicate that in infected cells, gamma(1)34.5 interacts with and redirects phosphatase to dephosphorylate eIF-2alpha to enable continued protein synthesis despite the presence of activated PKR. The GADD34 protein may have a similar function in eukaryotic cells. The proposed mechanism for maintenance of protein synthesis in the face of double-stranded RNA accumulation is different from that described for viruses examined to date.
Figures
Figure 1
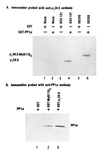
Association of PP1α with γ134.5 and MyD116. (A) Replicate HeLa cell cultures were harvested in lysis buffer containing 10 mM Hepes (pH 7.6), 250 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and 2 mM benzamidine 18 hr after mock infection or infection with 10 pfu of HSV-1(F) or R8300 per cell. After 30 min on wet ice and low speed centrifugation to remove nuclei, the supernatant fluids were precleared with GST beads and then reacted with GST phosphatase 1 bound beads at 4°C overnight. After extensive rinsing, the proteins bound to beads were solubilized by boiling in disruption buffer containing 50 mM Tris·HCl (pH 7.0), 5% 2-mercaptoethanol, 2% SDS, and 2.75% sucrose, electrophoretically separated on denaturing 12% polyacrylamide gels, and transferred to a nitrocellulose sheet, and the blot was probed with anti-γ134.5 serum (1). The positions of γ134.5 protein and of the chimeric protein γ134.5–MyD116 are shown. (B) An aliquot of GST–PP1α fusion protein bound to beads was reacted with 25 units of thrombin (Sigma) in PBS at room temperature. After 8 hr, the mixture was spun in a table top centrifuge and the supernatant fluid containing PP1α was then dialyzed against lysis buffer, reacted with GST, GST–MyD116C, or GST–γ134.5C bound to beads and processed as described in A. PP1 was detected with antiphosphatase 1α antibody (Upstate Biotechnology, Lake Placid, NY).
Figure 2
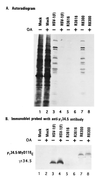
(A) Autoradiographic images of electrophoretically separated, [35S]methionine-labeled proteins from cell lysates infected with the indicated viruses in the presence or absence of okadaic acid (OA). SK-N-SH cells were either mock-infected or infected with 20 pfu of HSV-1(F), R3616, or R8300 per cell. At 2 hr after exposure to the viruses, the cells were overlaid with medium 199V (1) supplemented with or without okadaic acid (25 ng/ml). At 14 hr after infection, the cells were overlaid for 1 hr with 1 ml of medium 199V lacking methionine but supplemented with 50 μCi of [35S]methionine (specific activity, >1000 Ci/mmol; Amersham) for 1 hr, then harvested, solubilized, subjected to electrophoresis in denaturing 12% polyacrylamide gels, transferred to a nitrocellulose sheet, and subjected to autoradiography as described (6). (B) Immunoblot of the nitrocellulose sheet in A probed with anti-γ134.5 antibody (1).
Figure 3
Autoradiographic images of purified, electrophoretically separated eIF-2 after reaction with various amounts of S10 fractions of HeLa cells mock-infected or infected with HSV-1(F), R3616, or R8300. The assays were carried out as described. The amount of S10 fractions used are indicated on the top. Arrows marked α and β indicate the position of the α and β subunits of eIF-2; the arrow marked 39K indicates the position of a trace 39-kDa protein unrelated to eIF-2α.
Figure 4
Phosphatase activity in S10 fractions of HeLa cells mock-infected or infected with 20 pfu of HSV-1(F), R3616, or R8300 per cell. The procedures were carried out as described. (A) Fraction of residual radioactivity in eIF-2α-32P after reaction with variable amounts of S10 fractions from mock-infected or infected cells. (B) Effect of inhibitor 2 on the rate of dephosphorylation of eIF-2α-32P by the phosphatase activity in the S10 fractions. The results are expressed as the relative rate of activity seen in duplicate samples containing no added inhibitor 2. (C) [32P]phosphorylase phosphatase activity. The procedure was the same as in A except that the dephosphorylation of [32P]phosphorylase by the S10 fractions was assayed. The results are an average of two determinations and are expressed as the percentage of [32P]phosphorylase remaining, when compared with zero time samples. (D) Effect of inhibitor 2 on the relative rate of [32P]phosphorylase phosphatase activity. The assay was done as described in B.
Figure 5
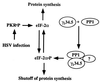
Schematic representation of the regulation of protein synthesis by γ134.5 in infected cells. As described in the text, PKR is activated after the onset of DNA synthesis in cells infected with HSV-1. In the absence of γ134.5, eIF-2α is phosphorylated and protein synthesis ceases. γ134.5 binds to PP1α and dephosphorylates eIF-2α. It is not known whether the γ134.5–PP1 complex is sufficient or requires additional factors to dephosphorylate eIF-2α. Because GADD34 substitutes for γ134.5, it is conceivable that one of the functions of GADD34 is to prevent shutoff of protein synthesis by a similar mechanism.
Similar articles
- The eIF-2alpha kinases and the control of protein synthesis.
de Haro C, Méndez R, Santoyo J. de Haro C, et al. FASEB J. 1996 Oct;10(12):1378-87. doi: 10.1096/fasebj.10.12.8903508. FASEB J. 1996. PMID: 8903508 Review.
Cited by
- Neoadjuvant oncolytic virus orienx010 and toripalimab in resectable acral melanoma: a phase Ib trial.
Liu J, Wang X, Li Z, Gao S, Mao L, Dai J, Li C, Cui C, Chi Z, Sheng X, Lai Y, Tan Z, Lian B, Tang B, Yan X, Li S, Zhou L, Wei X, Li J, Guo J, Si L. Liu J, et al. Signal Transduct Target Ther. 2024 Nov 22;9(1):318. doi: 10.1038/s41392-024-02029-2. Signal Transduct Target Ther. 2024. PMID: 39572525 Free PMC article. Clinical Trial. - Advancements, challenges, and future perspectives in developing feline herpesvirus 1 as a vaccine vector.
Luo X, Liang R, Liang L, Tang A, Hou S, Ding J, Li Z, Tang X. Luo X, et al. Front Immunol. 2024 Sep 12;15:1445387. doi: 10.3389/fimmu.2024.1445387. eCollection 2024. Front Immunol. 2024. PMID: 39328406 Free PMC article. Review. - The emerging field of viroimmunotherapy for pediatric brain tumors.
Garcia-Moure M, Laspidea V, Gupta S, Gillard AG, Khatua S, Parthasarathy A, He J, Lang FF, Fueyo J, Alonso MM, Gomez-Manzano C. Garcia-Moure M, et al. Neuro Oncol. 2024 Nov 4;26(11):1981-1993. doi: 10.1093/neuonc/noae160. Neuro Oncol. 2024. PMID: 39148489 Review. - Advances in the immunoescape mechanisms exploited by alphaherpesviruses.
Wang Y, Ma C, Wang S, Wu H, Chen X, Ma J, Wang L, Qiu HJ, Sun Y. Wang Y, et al. Front Microbiol. 2024 Jun 19;15:1392814. doi: 10.3389/fmicb.2024.1392814. eCollection 2024. Front Microbiol. 2024. PMID: 38962133 Free PMC article. Review. - The immune response to RNA suppresses nucleic acid synthesis by limiting ribose 5-phosphate.
Bhattacharjee P, Wang D, Anderson D, Buckler JN, de Geus E, Yan F, Polekhina G, Schittenhelm R, Creek DJ, Harris LD, Sadler AJ. Bhattacharjee P, et al. EMBO J. 2024 Jul;43(13):2636-2660. doi: 10.1038/s44318-024-00100-w. Epub 2024 May 22. EMBO J. 2024. PMID: 38778156 Free PMC article.
References
- Chou J, Kern E R, Whitely R J, Roizman B. Science. 1990;250:1262–1266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases