NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion - PubMed (original) (raw)
NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion
A I Barth et al. J Cell Biol. 1997.
Abstract
beta-Catenin is essential for the function of cadherins, a family of Ca2+-dependent cell-cell adhesion molecules, by linking them to (alpha)-catenin and the actin cytoskeleton. beta-Catenin also binds to adenomatous polyposis coli (APC) protein, a cytosolic protein that is the product of a tumor suppressor gene mutated in colorectal adenomas. We have expressed mutant beta-catenins in MDCK epithelial cells to gain insights into the regulation of beta-catenin distribution between cadherin and APC protein complexes and the functions of these complexes. Full-length beta-catenin, beta-catenin mutant proteins with NH2-terminal deletions before (deltaN90) or after (deltaN131, deltaN151) the alpha-catenin binding site, or a mutant beta-catenin with a COOH-terminal deletion (delta C) were expressed in MDCK cells under the control of the tetracycline-repressible transactivator. All beta-catenin mutant proteins form complexes and colocalize with E-cadherin at cell-cell contacts; deltaN90, but neither deltaN131 nor deltaN151, bind alpha-catenin. However, beta-catenin mutant proteins containing NH2-terminal deletions also colocalize prominently with APC protein in clusters at the tips of plasma membrane protrusions; in contrast, full-length and COOH-terminal-deleted beta-catenin poorly colocalize with APC protein. NH2-terminal deletions result in increased stability of beta-catenin bound to APC protein and E-cadherin, compared with full-length beta-catenin. At low density, MDCK cells expressing NH2-terminal-deleted beta-catenin mutants are dispersed, more fibroblastic in morphology, and less efficient in forming colonies than parental MDCK cells. These results show that the NH2 terminus, but not the COOH terminus of beta-catenin, regulates the dynamics of beta-catenin binding to APC protein and E-cadherin. Changes in beta-catenin binding to cadherin or APC protein, and the ensuing effects on cell morphology and adhesion, are independent of beta-catenin binding to alpha-catenin. These results demonstrate that regulation of beta-catenin binding to E-cadherin and APC protein is important in controlling epithelial cell adhesion.
Figures
Figure 1
Schematic representation of KT3-tagged full-size and mutant β-catenin proteins. NH2- and COOH-terminal domains and the 13 internal armadillo-like repeats of β-catenin are indicated. A stretch of unrepeated amino acids between repeat 10 and 11 (empty box) is shown. The binding sites for α-catenin, E-cadherin, APC, and the epitopes for rabbit β-catenin antisera β-cat.N, β-cat.C, and mouse mAb KT3 are indicated. The epitope for antiserum β-cat.N is deleted in ΔN90, ΔN131, and ΔN151, and these mutant proteins are not detected by β-cat.N. A mouse mAb raised against the 212 COOH-terminal amino acids of β-catenin was used instead of antiserum β-cat.C, and both β-cat.C antibodies do not detect ΔC. The following amino acids are deleted in the mutants: ΔC, 696–781; ΔN90, 1–90; ΔN131, 1–131; ΔN151, 1–151.
Figure 2
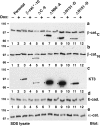
Dox-repressible expression of β-catenin mutant proteins in MDCK cells. MDCK clones were cultured for 4 d without or with Dox (−/+ Dox) and extracted with 1% SDS. 15-μg protein lysates were subjected to SDS-PAGE and immunoblotted with different antibodies (see Fig. 1). β-catenin mutant proteins (stars in a–c) were expressed only in cultures without Dox; endogenous β-catenin was expressed in all cultures (a and b). Expression levels of exogenous full-length β-catenin*, ΔN90, ΔN131, and ΔN151 were compared with that of endogenous β-catenin by immunoblotting with mAb β-cat.C (a). Exogenous β-catenin* and ΔC were compared with endogenous β-catenin by immunoblotting with β-cat.N (b). The expression levels of mutant β-catenin proteins in different clones were compared by immunoblotting with the tag antibody KT3 (c). The β-cat.C blot was reblotted with E-cadherin antiserum (d). The KT3 blot was reblotted with α-catenin antiserum (e). Molecular mass standards are indicated in kD.
Figure 3
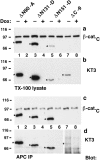
β-catenin mutant proteins compete with endogenous β-catenin for binding to E-cadherin and α-catenin. MDCK clones were cultured 4 d without or with Dox (−/+) and extracted with 1% Triton X-100 lysis buffer. Protein lysates were split: 400-μg lysates were immunoprecipitated with E-cadherin antiserum to analyze binding of mutant β-catenin proteins to E-cadherin (a and b). 400 μg protein lysates were immunoprecipitated with α-catenin antiserum to analyze binding of mutant β-catenin proteins to α-catenin (c and d). Equivalent fractions of the immunoprecipitates were subjected to SDS-PAGE and blotted with mAbs KT3 (a and c) or β-cat.C (b and d). β-catenin mutant proteins are indicated (stars).
Figure 4
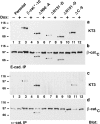
ΔN90, ΔN131, and ΔN151 are enriched in APC protein complexes. Aliquots of the same Triton X-100 lysates as described in Fig. 3 were either subjected to SDS-PAGE or used for immunoprecipitation with APC antiserum (a–d). 9-μg protein lysates were subjected to SDS-PAGE and immunoblotted with the indicated mAbs to compare the ratio of β-catenin mutant proteins to endogenous β-catenin (a), and to each other (b). 1,500-μg protein lysates were immunoprecipitated with APC antiserum. Equivalent fractions of the immunoprecipitates were subjected to SDS-PAGE and blotted with the indicated mAbs to analyze binding of mutant β-catenin proteins to APC (c–d). β-catenin mutant proteins are indicated (stars). A longer exposure of the blot in (d) is shown for lanes 7 and 8. Triton X-100 lysates were prepared from clones β-catenin*–7 and ΔN131-7, and aliquots of the lysates were immunoprecipitated with antiserum β-cat.C or APC antiserum (e). Immunoprecipitates were subjected to SDSPAGE and blotted with antiserum β-cat.C to analyze the ratio of exogenous β-catenin* and ΔN131 to endogenous β-catenin. β-catenin mutant proteins are indicated (stars).
Figure 4
ΔN90, ΔN131, and ΔN151 are enriched in APC protein complexes. Aliquots of the same Triton X-100 lysates as described in Fig. 3 were either subjected to SDS-PAGE or used for immunoprecipitation with APC antiserum (a–d). 9-μg protein lysates were subjected to SDS-PAGE and immunoblotted with the indicated mAbs to compare the ratio of β-catenin mutant proteins to endogenous β-catenin (a), and to each other (b). 1,500-μg protein lysates were immunoprecipitated with APC antiserum. Equivalent fractions of the immunoprecipitates were subjected to SDS-PAGE and blotted with the indicated mAbs to analyze binding of mutant β-catenin proteins to APC (c–d). β-catenin mutant proteins are indicated (stars). A longer exposure of the blot in (d) is shown for lanes 7 and 8. Triton X-100 lysates were prepared from clones β-catenin*–7 and ΔN131-7, and aliquots of the lysates were immunoprecipitated with antiserum β-cat.C or APC antiserum (e). Immunoprecipitates were subjected to SDSPAGE and blotted with antiserum β-cat.C to analyze the ratio of exogenous β-catenin* and ΔN131 to endogenous β-catenin. β-catenin mutant proteins are indicated (stars).
Figure 5
Increased stability of ΔN90, ΔN131, and ΔN151 in the E-cadherin– and APC protein–bound pools. MDCK clones were cultured 0, 6, 12, or 18 h with Dox and extracted with 1% Triton X-100 lysis buffer. Protein lysates were split: 1,500-μg protein lysates were immunoprecipitated with APC antiserum (first column), and 500-μg lysates were immunoprecipitated with E-cadherin antiserum (second column). Equivalent fractions of the immunoprecipitates and 50-μg (β-cat.*-10,-7, ΔC-9) or 25-μg (ΔN90-A, ΔN131-D, ΔN151-D) protein lysates (third column) were subjected to SDS-PAGE and immunoblotted with mAb KT3. For β-catenin*–10, –7, and ΔC-9 blots, three times more of the APC immunoprecipitations were used than for the ΔN90-A, ΔN131-D, and ΔN151-D blots, and the blots were exposed longer.
Figure 6
ΔN151, ΔN131, and ΔN90 localize to clusters near the plasma membrane in extending membranes of MDCK cells. MDCK clones were double stained with mAb KT3 against the epitope tag in β-catenin mutant proteins (a–e) and antiserum against E-cadherin (a′), antiserum β-cat.N against endogenous β-catenin (b′), and antiserum against α-catenin (c′–e′). Areas with ΔN151, ΔN131, and ΔN90 clusters are indicated by arrowheads (a–d). These clusters are not detected with E-cadherin, endogenous β-catenin, or α-catenin antisera (a′–d′, arrowheads). ΔN131 expression was repressed in ΔN131-D by 48 h incubation with Tet before fixation (e). (Arrows) Areas of intercellular contact that are stained by antisera to E-cadherin (a′), endogenous β-catenin (b′), α-catenin (c′–e′), and sometimes weakly by mAb KT3 (a–d). Bar, 10 μm.
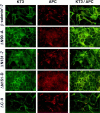
Figure 7
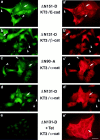
ΔN90, ΔN131, and ΔN151 colocalize with APC protein in MDCK cells. MDCK clones were double stained with mAb KT3 against the epitope tag in β-catenin mutant proteins and antiserum against APC protein. Full-length β-catenin* and ΔC localize to the lateral membranes of cells and show little overlap with APC protein clusters (top and bottom panel). ΔN90, ΔN131, and ΔN151 localize to lateral membranes of cells and colocalize with APC protein in clusters (middle panels). Bar, 10 μm.
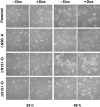
Figure 8
Effect of ΔN90, ΔN131, and ΔN151 expression on colony formation in low density MDCK cultures. Cultures of MDCK clones were untreated or pretreated 3 d with Dox and plated at low density without (−) or with (+) Dox. Cell morphology was analyzed 24 and 48 h after plating. Expression of ΔN90, ΔN131, and ΔN151 delayed formation of tight round colonies in low density cultures (first and third columns). This effect could be reversed by repression of expression of ΔN90, ΔN131, and ΔN151 with Dox (second and fourth columns). Dox itself had no effect on the morphology of the parental cells (top panel). Bar, 40 μm.
Similar articles
- Dynamics of beta-catenin interactions with APC protein regulate epithelial tubulogenesis.
Pollack AL, Barth AI, Altschuler Y, Nelson WJ, Mostov KE. Pollack AL, et al. J Cell Biol. 1997 Jun 30;137(7):1651-62. doi: 10.1083/jcb.137.7.1651. J Cell Biol. 1997. PMID: 9199178 Free PMC article. - Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells.
Efstathiou JA, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, Hattori T, Wright NA, Bodmer WF, Pignatelli M. Efstathiou JA, et al. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3122-7. doi: 10.1073/pnas.95.6.3122. Proc Natl Acad Sci U S A. 1998. PMID: 9501226 Free PMC article. - The expression of E-cadherin and catenins in colorectal tumours from familial adenomatous polyposis patients.
El-Bahrawy MA, Talbot IC, Poulsom R, Jeffery R, Alison MR. El-Bahrawy MA, et al. J Pathol. 2002 Sep;198(1):69-76. doi: 10.1002/path.1168. J Pathol. 2002. PMID: 12210065 - Adenomatous polyposis coli (Apc) tumor suppressor gene as a multifunctional gene.
Senda T, Shimomura A, Iizuka-Kogo A. Senda T, et al. Anat Sci Int. 2005 Sep;80(3):121-31. doi: 10.1111/j.1447-073x.2005.00106.x. Anat Sci Int. 2005. PMID: 16158975 Review. - Cadherin-catenin complex: protein interactions and their implications for cadherin function.
Aberle H, Schwartz H, Kemler R. Aberle H, et al. J Cell Biochem. 1996 Jun 15;61(4):514-23. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. J Cell Biochem. 1996. PMID: 8806074 Review.
Cited by
- Wnt activation disturbs cell competition and causes diffuse invasion of transformed cells through NF-κB-MMP21 pathway.
Nakai K, Lin H, Yamano S, Tanaka S, Kitamoto S, Saitoh H, Sakuma K, Kurauchi J, Akter E, Konno M, Ishibashi K, Kamata R, Ohashi A, Koseki J, Takahashi H, Yokoyama H, Shiraki Y, Enomoto A, Abe S, Hayakawa Y, Ushiku T, Mutoh M, Fujita Y, Kon S. Nakai K, et al. Nat Commun. 2023 Nov 3;14(1):7048. doi: 10.1038/s41467-023-42774-6. Nat Commun. 2023. PMID: 37923722 Free PMC article. - Microglial depletion after brain injury prolongs inflammation and impairs brain repair, adult neurogenesis and pro-regenerative signaling.
Palsamy K, Chen JY, Skaggs K, Qadeer Y, Connors M, Cutler N, Richmond J, Kommidi V, Poles A, Affrunti D, Powell C, Goldman D, Parent JM. Palsamy K, et al. Glia. 2023 Nov;71(11):2642-2663. doi: 10.1002/glia.24444. Epub 2023 Jul 14. Glia. 2023. PMID: 37449457 Free PMC article. - Co-activation of Sonic hedgehog and Wnt signaling in murine retinal precursor cells drives ocular lesions with features of intraocular medulloepithelioma.
Dottermusch M, Sumisławski P, Krevet J, Middelkamp M, Voß H, Siebels B, Bartsch H, Sotlar K, Meyer P, Frank S, Korshunov A, Glatzel M, Schüller U, Neumann JE. Dottermusch M, et al. Oncogenesis. 2021 Nov 16;10(11):78. doi: 10.1038/s41389-021-00369-0. Oncogenesis. 2021. PMID: 34785636 Free PMC article. - An APC Mutation in a Large Chinese Kindred With Familial Adenomatous Polyposis Was Identified Using Both Next Generation Sequencing and Simple STR Marker Haplotypes.
Zhan Q, Wang L, Xu X, Sun Y, Li L, Qi X, Chen F, Wei X, Raff ML, Yu P, Jin F. Zhan Q, et al. Front Genet. 2020 Mar 4;11:191. doi: 10.3389/fgene.2020.00191. eCollection 2020. Front Genet. 2020. PMID: 32194643 Free PMC article. - PSPC1-interchanged interactions with PTK6 and β-catenin synergize oncogenic subcellular translocations and tumor progression.
Lang YD, Chen HY, Ho CM, Shih JH, Hsu EC, Shen R, Lee YC, Chen JW, Wu CY, Yeh HW, Chen RH, Jou YS. Lang YD, et al. Nat Commun. 2019 Dec 16;10(1):5716. doi: 10.1038/s41467-019-13665-6. Nat Commun. 2019. PMID: 31844057 Free PMC article.
References
- Aberle, H., S. Butz, J. Stappert, H. Weissig, R. Kemler, and H. Hoschuetzky. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 3655–3663. - PubMed
- Aberle H, Schwartz H, Hoschuetzky H, Kemler R. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to α-catenin. J Biol Chem. 1996;271:1520–1526. - PubMed
- Butz S, Stappert J, Weissig H, Kemler R. Plakoglobin and betacatenin: distinct but closely related [letter] Science (Wash DC) 1992;257:1142–1144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous