Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes - PubMed (original) (raw)
Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes
Y Feng et al. J Neurosci. 1997.
Abstract
Fragile X syndrome, a leading cause of inherited mental retardation, is attributable to the unstable expansion of a CGG-repeat within the FMR1 gene that results in the absence of the encoded protein. The fragile X mental retardation protein (FMRP) is a ribosome-associated RNA-binding protein of uncertain function that contains nuclear localization and export signals. We show here detailed cellular localization studies using both biochemical and immunocytochemical approaches. FMRP was highly expressed in neurons but not glia throughout the rat brain, as detected by light microscopy. Although certain structures, such as hippocampus, revealed a strong signal, the regional variation in staining intensity appeared to be related to neuron size and density. In human cell lines and mouse brain, FMRP co-fractionated primarily with polysomes and rough endoplasmic reticulum. Ultrastructural studies in rat brain revealed high levels of FMRP immunoreactivity in neuronal perikarya, where it is concentrated in regions rich in ribosomes, particularly near or between rough endoplasmic reticulum cisternae. Immunogold studies also provided evidence of nucleocytoplasmic shuttling of FMRP, which was localized in neuronal nucleoplasm and within nuclear pores. Moreover, labeling was observed in large- and small-caliber dendrites, in dendritic branch points, at the origins of spine necks, and in spine heads, all known locations of neuronal polysomes. Dendritic localization, which was confirmed by co-fractionation of FMRP with synaptosomal ribosomes, suggests a possible role of FMRP in the translation of proteins involved in dendritic structure or function and relevant for the mental retardation occurring in fragile X syndrome.
Figures
Fig. 1.
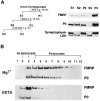
FMRP distribution in subcellular fractions of human lymphoblastoid cells. A is a schematic illustration of subcellular fractionation of EBV-transformed human lymphoblastoid cells. The descriptor under key fractions refers to the panels below(B_–_D), with INT as interface. A detailed description and protocol is provided in Materials and Methods. In_B_, the left panel shows SDS-PAGE immunoblot analysis of FMRP and P0 in crude subcellular fractions. Total protein (3 μg) from each fraction of B1–B5 was loaded. Densitometric analysis of immunoblot signals was used to calculate the total yield of FMRP in each corresponding fraction, based on the total fraction volume. The relative percentage of FMRP in each fraction was plotted as shown in the right panel. _C_shows the SDS-PAGE immunoblot analysis of FMRP and P0 in separated postnuclear supernatant fraction. C1–C4 in sucrose gradient fractionation 1 represent cytosol; low-density membranes (plasma membrane, Golgi, and smooth ER); high-density membranes (RER); and free polysome pellet, respectively. Total protein (1.5 μg) from each fraction was loaded. D shows the SDS-PAGE immunoblot analysis of FMRP and P0 in various ER components.D1–D3 in sucrose gradient fractionation 2 represent smooth ER, light RER, and heavy RER. Total protein (3 μg) from each fraction was loaded.
Fig. 2.
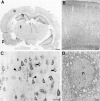
FMRP immunocytochemistry in the rat brain.A is a coronal section demonstrating widespread FMRP labeling. The most intense labeling is in the cellular layers of the hippocampus (h) and pyriform cortex (p), which are regions with extremely high neuronal densities. The deeper layers of the cerebral cortex (c) are also well labeled. B demonstrates FMRP immunoreactivity in the frontal cortex at higher magnification. It appears that most neurons in each cortical layer are FMRP-positive.C illustrates the cellular pattern of FMRP immunoreactivity in layer V pyramidal cells from frontal cortex. Staining is very dense in perikarya and proximal dendrites (triangles). In contrast, nuclear staining (arrows) is uncertain. D is an electron micrograph of the soma of a cerebral cortical pyramidal cell. With immunoperoxidase, dense cytoplasmic staining is evident. Although the nucleus is somewhat dark, FMRP immunoreactivity is not clearly present. Scale bars: A, 50 mm; B, 100 μm;C, 50 μm; D, 1 μm.
Fig. 3.
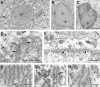
A, Electron micrograph of the soma of a labeled cerebral cortical pyramidal neuron. Immunogold particles are present both in the nucleus (n) and perikaryon. Little of the cytoplasmic label is associated with the plasma membrane, mitochondria, or Golgi apparatus (arrow).B, C, Few particles were found in the nuclei or cytoplasm of astrocytes (a) or oligodendrocytes (o). D, Tangential section through the nuclear envelope of a pyramidal cell showing three nuclear pores (arrows), one of which (longer arrow) contains an immunogold particle. E, Cross-section through the nuclear envelope showing immunogold particles within nuclear pores (arrows).F_–_H, In perikarya, most immunogold particles are clustered between the cisternae of RER. These regions are especially rich in free ribosomes, visible here as fine electron-dense particles (asterisks). A few immunogold particles are also seen in direct contact with the cisternae (arrows). Scale bars: A_–_C, 1 μm;D_–_H, 500 μm.
Fig. 4.
Electron micrographs demonstrating FMRP localization in cellular processes in cerebral cortex.A_–_C, Dendrites (d) in cross-section and longitudinal section showing that immunogold particles are either free in the cytoplasm or clustered around cisternae of smooth ER (arrows) or at the origins of dendritic spines (triangles).D_–_F, Dendritic spines (s) containing immunogold particles, which are either free in the cytoplasm or associated with the spine apparatus (arrows).G, Rare axon terminals (a) contain immunogold particles that are cytoplasmic in location.H, FMRP-immunoreactive axon terminals (a) are more easily identified using immunoperoxidase. Scale bars:A_–_H, 500 nm.
Fig. 5.
Association of FMRP with translating ribosomes in rat cortex. A, Subcellular fractionation of rat cortex by velocity centrifugation. The fractionation procedures are illustrated on the left panel, with S_indicating supernatant and P indicating pellet. A detailed description and protocol are provided in Materials and Methods. The right panel shows SDS-PAGE immunoblot analysis of FMRP and other marker proteins in the corresponding fractions as indicated. Based on Bradford assay, 20 μg of total protein from each fraction was used in this blot. B, Association of FMRP with polysomes in synaptosomal lysate. SDS-PAGE immunoblot analysis was performed using linear sucrose gradient fractions containing synaptosomal lysates with the presence of Mg2+ or EDTA, as described in Materials and Methods. The signals of FMRP and P0 protein are indicated on the_right. The sedimentation of ribosomal components in human lymphoblasts monitored at OD254 in a parallel gradient are indicated on top of the corresponding fractions.
Similar articles
- Association of FMRP with ribosomal precursor particles in the nucleolus.
Willemsen R, Bontekoe C, Tamanini F, Galjaard H, Hoogeveen A, Oostra B. Willemsen R, et al. Biochem Biophys Res Commun. 1996 Aug 5;225(1):27-33. doi: 10.1006/bbrc.1996.1126. Biochem Biophys Res Commun. 1996. PMID: 8769090 - NUFIP1 (nuclear FMRP interacting protein 1) is a nucleocytoplasmic shuttling protein associated with active synaptoneurosomes.
Bardoni B, Willemsen R, Weiler IJ, Schenck A, Severijnen LA, Hindelang C, Lalli E, Mandel JL. Bardoni B, et al. Exp Cell Res. 2003 Sep 10;289(1):95-107. doi: 10.1016/s0014-4827(03)00222-2. Exp Cell Res. 2003. PMID: 12941608 - Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses.
Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Antar LN, et al. J Neurosci. 2004 Mar 17;24(11):2648-55. doi: 10.1523/JNEUROSCI.0099-04.2004. J Neurosci. 2004. PMID: 15028757 Free PMC article. - Molecular insights into mental retardation: multiple functions for the Fragile X mental retardation protein?
Zalfa F, Bagni C. Zalfa F, et al. Curr Issues Mol Biol. 2004 Jul;6(2):73-88. Curr Issues Mol Biol. 2004. PMID: 15119819 Review. - The fragile X syndrome.
Hoogeveen AT, Oostra BA. Hoogeveen AT, et al. J Inherit Metab Dis. 1997 Jun;20(2):139-51. doi: 10.1023/a:1005392319533. J Inherit Metab Dis. 1997. PMID: 9211186 Review.
Cited by
- Region-Related Differences in Short-Term Synaptic Plasticity and Synaptotagmin-7 in the Male and Female Hippocampus of a Rat Model of Fragile X Syndrome.
Tsotsokou G, Miliou A, Trompoukis G, Leontiadis LJ, Papatheodoropoulos C. Tsotsokou G, et al. Int J Mol Sci. 2024 Jun 26;25(13):6975. doi: 10.3390/ijms25136975. Int J Mol Sci. 2024. PMID: 39000085 Free PMC article. - The Role of NRF2 in Trinucleotide Repeat Expansion Disorders.
Chang KH, Chen CM. Chang KH, et al. Antioxidants (Basel). 2024 May 26;13(6):649. doi: 10.3390/antiox13060649. Antioxidants (Basel). 2024. PMID: 38929088 Free PMC article. Review. - Fragile X Messenger Ribonucleoprotein Protein and Its Multifunctionality: From Cytosol to Nucleolus and Back.
Taha MS, Ahmadian MR. Taha MS, et al. Biomolecules. 2024 Mar 26;14(4):399. doi: 10.3390/biom14040399. Biomolecules. 2024. PMID: 38672417 Free PMC article. Review. - Translational modulator ISRIB alleviates synaptic and behavioral phenotypes in Fragile X syndrome.
Coulson RL, Frattini V, Moyer CE, Hodges J, Walter P, Mourrain P, Zuo Y, Wang GX. Coulson RL, et al. iScience. 2024 Feb 16;27(4):109259. doi: 10.1016/j.isci.2024.109259. eCollection 2024 Apr 19. iScience. 2024. PMID: 38510125 Free PMC article. - Advances in the understanding of the pathophysiology of schizophrenia and bipolar disorder through induced pluripotent stem cell models.
Perrottelli A, Marzocchi FF, Caporusso E, Giordano GM, Giuliani L, Melillo A, Pezzella P, Bucci P, Mucci A, Galderisi S. Perrottelli A, et al. J Psychiatry Neurosci. 2024 Mar 15;49(2):E109-E125. doi: 10.1503/jpn.230112. Print 2024 Jan-Feb. J Psychiatry Neurosci. 2024. PMID: 38490647 Free PMC article. Review.
References
- Abitbol M, Menini C, Delezoide A-L, Rhyner T, Vekemans M, Mallet J. Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nat Genet. 1993;4:147–153. - PubMed
- Ashley C, Wilkinson K, Reines D, Warren S. FMR 1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. - PubMed
- Bonfa E, Parnassa AP, Rhoads DD, Roufa DJ, Wool IG, Elkon KB. Antiribosomal S10 antibodies in humans and MRL/lpr mice with systemic lupus erythematosus. Arthritis Rheum. 1989;32:1252–1261. - PubMed
- Brown WT, Houck GE, Jeziorowska A, Levinson FN, Ding X, Dobkin C, Zhong N, Henderson J, Sklower Brooks S, Jenkins EC. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. J Am Med Assoc. 1993;270:1569–1575. - PubMed
- Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases