Dendritic and postsynaptic localizations of glycine receptor alpha subunit mRNAs - PubMed (original) (raw)
Dendritic and postsynaptic localizations of glycine receptor alpha subunit mRNAs
C Racca et al. J Neurosci. 1997.
Abstract
Some synaptic neurotransmitter receptors, such as those for glycine, have somato-dendritic distributions. Although the machinery for protein synthesis and several mRNAs are present in dendrites and close to synapses in central neurons, so far the mRNAs for neurotransmitter receptors have not been found unequivocally in dendrites. The glycine receptor (GlyR), a ligand-gated channel mediating a chloride-dependent inhibition, is composed of transmembrane alpha and beta subunits. GlyRs are only present at glycinergic postsynaptic differentiation, where they are stabilized by the associated protein gephyrin. With light nonradioactive in situ hybridization (ISH), we observe that GlyR alpha subunit mRNAs are present in both somata and dendrites of most neurons of the ventral horn of rat spinal cord, whereas the beta subunit and gephyrin mRNAs are predominantly in somata. Interestingly, within dendrites GlyR alpha subunit mRNAs form aggregates that are mostly localized peripherally to the dendritic axial core. Electron microscopic ISH shows that GlyR alpha subunit mRNAs are associated with postsynaptic differentiations. At these sites, the GlyR alpha subunit mRNAs are detected in close association with subsynaptic cisternae. This targeting of alpha subunit mRNAs to postsynaptic domains could provide a means of dynamically modulating synaptic efficacy by changing the composition and the density of receptors at glycinergic synapses.
Figures
Fig. 1.
Localization of GlyR subunit and gephyrin mRNAs in ventral horn spinal cord neurons revealed by alkaline phosphatase enzymatic reaction product. Glycine receptor α1 (A) and α2 (B) mRNAs are detected in both somata (arrows) and neurites (arrowheads), whereas GlyR β subunit (C) and gephyrin (D) mRNAs are predominantly in the cell bodies (arrows). All examples are from the same animal and experiment. Photos were processed identically. Scale bar, 100 μm.
Fig. 2.
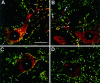
Double-fluorescence labeling of GlyR and gephyrin mRNAs and of the corresponding proteins detected with confocal microscopy. In each case, the mRNA signals are red, and the immunoreactivities for GlyR (mAb 4a) and gephyrin (mAB 7a) proteins are green. A, B, Presence of α1 (A) and α2 (B) mRNAs in somata and dendrites. Note their accumulation at dendritic branch points (arrowheads). Cross sections of dendrites (B; crossed arrows) containing α2 mRNAs. C, D, GlyR β subunit (C) and gephyrin (D) mRNAs are predominantly in the somata. The mRNA signals are red, and the immunoreactivities for GlyR and gephyrin proteins are_green_. The postsynaptic GlyR (A_–_C) and gephyrin (D) immunoreactivities (arrows) delineate neurons and dendrites. The nuclei (n) are not stained. Pixel size, 0.2 μm. Scale bar, 25 μm.
Fig. 3.
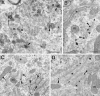
Subcellular localization of GlyR α1 subunit mRNAs in the somata of central horn neurons. A, Uneven distribution of transcripts visualized with HRP reaction product (arrowheads), which is frequently associated with cisternae (double arrowheads). Presence of an electron-dense HRP reaction product in front of a synaptic contact (arrow). B, Gold particles (arrowheads) associated with the mRNA are adjacent to a cisterna (double arrowhead). C, Lower magnification from the same neuron as in B.D, Further example showing the close relationship of the gold particles with cisternae of the Nissl bodies. Note that in_A_–C the Golgi apparatus (asterisks) are not decorated with either HRP reaction product or gold particles, respectively. m, Mithocondrium; n, nucleus. Scale bars: A,C, 0.5 μm; B, D, 0.25 μm.
Fig. 4.
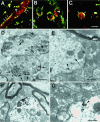
Dendritic and subsynaptic localization of GlyR α1 subunit mRNAs. A_–_C, Examples of optical sections of dendrites obtained with a confocal microscope (pixel size, 0.1 μm). In most cases, the mRNAs (red) were predominantly at the neurite periphery, here outlined by GlyR immunoreactivity (green; arrows), as seen on longitudinal (A) or transversal (B) views. Note that the mRNAs tend to form aggregates (arrowheads) within dendrites. In some dendrites (C), the mRNAs appear evenly distributed throughout the dendritic cross section. Colocalization of mRNA signal with GlyR immunoreactivity (yellow;A_–_C). D, EM ISH of mRNAs visualized in dendrites by HRP reaction product showing their tendency to accumulate peripherally to the dendritic center, and in front of synapses (arrows). Within dendrites, the mRNA signal is discontinuous and forms aggregates (arrowheads).E, The mRNAs detected by gold labeling (arrowheads) at the dendritic periphery are next to synapses. F, Decoration of the postsynaptic density by the HRP enzymatic reaction product. Note the presence of small subsynaptic cisternae (crossed arrow). G, Presence of gold-labeled mRNAs (arrowhead) on a minute cisterna, postsynaptic to a bouton containing pleomorphic vesicles. Scale bars: A_–_C, 5 μm;D, 1 μm; E, F, 0.5 μm;G, 0.25 μm.
Fig. 5.
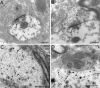
Gold particles associated to GlyR α1 subunit mRNA (arrowheads) are close to subsynaptic cisternae (crossed arrows). Examples at low-power (A1, B1) and high-power (A2, B2) magnifications, respectively.A2, Terminal bouton with pleomorphic vesicles (b). b, Terminal bouton. Scale bars:A1, B1, 0.5 μm; A2,B2, 0.25 μm.
Fig. 6.
Dendritic and subsynaptic localizations of GlyR α2 subunit mRNA. A, B, HRP reaction product (arrows) at postsynaptic densities and associated with submembranous cisternae (crossed arrows). C, D, Presence of gold particles (arrowheads) within a dendrite and beneath a terminal bouton (b1). Gold particles can be associated with cisternae (crossed arrows). b, Terminal bouton. Scale bars: A, B, 0.2 μm; C, 1 μm; D, 0.5 μm.
Similar articles
- Localization of components of glycinergic synapses during rat spinal cord development.
Colin I, Rostaing P, Augustin A, Triller A. Colin I, et al. J Comp Neurol. 1998 Aug 31;398(3):359-72. J Comp Neurol. 1998. PMID: 9714149 - Formation of glycine receptor clusters and their accumulation at synapses.
Meier J, Meunier-Durmort C, Forest C, Triller A, Vannier C. Meier J, et al. J Cell Sci. 2000 Aug;113 ( Pt 15):2783-95. doi: 10.1242/jcs.113.15.2783. J Cell Sci. 2000. PMID: 10893193 - A gephyrin-related mechanism restraining glycine receptor anchoring at GABAergic synapses.
Meier J, Grantyn R. Meier J, et al. J Neurosci. 2004 Feb 11;24(6):1398-405. doi: 10.1523/JNEUROSCI.4260-03.2004. J Neurosci. 2004. PMID: 14960612 Free PMC article. - Glycinergic transmission.
Kirsch J. Kirsch J. Cell Tissue Res. 2006 Nov;326(2):535-40. doi: 10.1007/s00441-006-0261-x. Epub 2006 Jun 29. Cell Tissue Res. 2006. PMID: 16807723 Review. - How to build a glycinergic postsynaptic membrane.
Betz H, Kuhse J, Schmieden V, Malosio ML, Langosch D, Prior P, Schmitt B, Kirsch J. Betz H, et al. J Cell Sci Suppl. 1991;15:23-5. doi: 10.1242/jcs.1991.supplement_15.4. J Cell Sci Suppl. 1991. PMID: 1668595 Review.
Cited by
- Spinal-Cord plasticity: independent and interactive effects of neuromodulator and activity-dependent plasticity.
Parker D. Parker D. Mol Neurobiol. 2000 Aug-Dec;22(1-3):55-80. doi: 10.1385/MN:22:1-3:055. Mol Neurobiol. 2000. PMID: 11414281 Review. - RNA Localization and Local Translation in Glia in Neurological and Neurodegenerative Diseases: Lessons from Neurons.
Blanco-Urrejola M, Gaminde-Blasco A, Gamarra M, de la Cruz A, Vecino E, Alberdi E, Baleriola J. Blanco-Urrejola M, et al. Cells. 2021 Mar 12;10(3):632. doi: 10.3390/cells10030632. Cells. 2021. PMID: 33809142 Free PMC article. Review. - Differential expression of TASK channels between horizontal interneurons and pyramidal cells of rat hippocampus.
Taverna S, Tkatch T, Metz AE, Martina M. Taverna S, et al. J Neurosci. 2005 Oct 5;25(40):9162-70. doi: 10.1523/JNEUROSCI.2454-05.2005. J Neurosci. 2005. PMID: 16207875 Free PMC article. - Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission.
Kittler JT, McAinsh K, Moss SJ. Kittler JT, et al. Mol Neurobiol. 2002 Oct-Dec;26(2-3):251-68. doi: 10.1385/MN:26:2-3:251. Mol Neurobiol. 2002. PMID: 12428759 Review. - The neuronal RNA binding protein Nova-1 recognizes specific RNA targets in vitro and in vivo.
Buckanovich RJ, Darnell RB. Buckanovich RJ, et al. Mol Cell Biol. 1997 Jun;17(6):3194-201. doi: 10.1128/MCB.17.6.3194. Mol Cell Biol. 1997. PMID: 9154818 Free PMC article.
References
- Ainger K, Hill S, Smith CL, Hill SJ, Barbarese E, Carson JH. Transport and localization of MBP mRNA within multicomponent granules in oligodendrocytes. Mol Biol Cell [Suppl] 1993b;4:421a.
- Altschuler RA, Betz H, Parakkal M, Reeks K, Wenthold R. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res. 1986;369:316–320. - PubMed
- Barbarese E, Koppel DE, Deutscher MP, Smith CL, Ainger K, Morgan F, Carson J. Protein translation components are colocalized in granules in oligodendrocytes. J Cell Sci. 1995;108:2781–2790. - PubMed
- Bassell GJ, Singer RH, Kosik KS. Association of poly(A) mRNA with microtubules in cultured neurons. Neuron. 1994;12:571–582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources