Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density - PubMed (original) (raw)
Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density
C S Woolley et al. J Neurosci. 1997.
Abstract
Previous studies have shown that estradiol induces new dendritic spines and synapses on hippocampal CA1 pyramidal cells. We have assessed the consequences of estradiol-induced dendritic spines on CA1 pyramidal cell intrinsic and synaptic electrophysiological properties. Hippocampal slices were prepared from ovariectomized rats treated with either estradiol or oil vehicle. CA1 pyramidal cells were recorded and injected with biocytin to visualize spines. The association of dendritic spine density and electrophysiological parameters for each cell was then tested using linear regression analysis. We found a negative relationship between spine density and input resistance; however, no other intrinsic property measured was significantly associated with dendritic spine density. Glutamate receptor autoradiography demonstrated an estradiol-induced increase in binding to NMDA, but not AMPA, receptors. We then used input/output (I/O) curves (EPSP slope vs stimulus intensity) to determine whether the sensitivity of CA1 pyramidal cells to synaptic input is correlated with dendritic spine density. Consistent with the lack of an estradiol effect on AMPA receptor binding, we observed no relationship between the slope of an I/O curve generated under standard recording conditions, in which the AMPA receptor dominates the EPSP, and spine density. However, recording the pharmacologically isolated NMDA receptor-mediated component of the EPSP revealed a significant correlation between I/O slope and spine density. These results indicate that, in parallel with estradiol-induced increases in spine/synapse density and NMDA receptor binding, estradiol treatment increases sensitivity of CA1 pyramidal cells to NMDA receptor-mediated synaptic input; further, sensitivity to NMDA receptor-mediated synaptic input is well correlated with dendritic spine density.
Figures
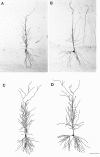
Fig. 1.
Photomicrographs of single sections containing a representative biocytin-filled CA1 pyramidal cell from an OVX+O (A) and an OVX+E (B) animal. Camera lucida tracings of the cell body and complete dendritic tree reconstructed from all sections containing the same cells from the OVX+O (C) or OVX+E (D) animal are shown in the_lower panels_. Camera lucida tracings were used to determine total dendritic length of each cell; no differences in total dendritic length were observed between treatment groups. Scale bar, 50 μm (applies to all panels).
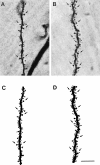
Fig. 2.
Photomicrographs of representative dendritic segments in the lateral branches of the apical dendritic tree from a CA1 pyramidal cell in an OVX+O (A) and an OVX+E (B) animal. Camera lucida tracings of the segment from the OVX+O (C) and OVX+E (D) cells show all visible dendritic spines. Some dendritic spines are indicated by_arrows_. Note the increased density of dendritic spines on the dendrites of the cell from an estradiol-treated animal. This estradiol-induced increase in spine density is quantified in Figure 3. Scale bar, 10 μm (applies to all panels).
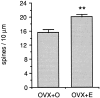
Fig. 3.
Bar graphs depicting the mean difference in dendritic spine density in the apical dendritic trees of CA1 pyramidal cells from ovariectomized, oil-treated (OVX+O), and ovariectomized, estradiol-treated (OVX+E) animals. All well filled CA1 pyramidal cells in this study were included. The difference in mean dendritic spine density is 22%; **p < 0.001.
Fig. 4.
Representative intracellular recordings from CA1 pyramidal cells in slices from an OVX+O (A) and OVX+E (B) animal. Recordings were made from the resting membrane potential in standard ACSF. In each panel, the _top traces_are voltage and the bottom traces are current. Calibration pulse, 10 mV, 10 msec. Note that input resistance is greater in the cell in A (OVX+O), which has a lower density of dendritic spines than the cell in B (OVX+E), which has higher spine density. C shows the negative correlation between input resistance and dendritic spine density in CA1 pyramidal cells. Data from cells in OVX+O (open squares), OVX+E (filled squares), and gonadally intact (open circles) animals are plotted together. Although there is a significant correlation between spine density and input resistance, there is no significant difference in mean input resistance between cells from OVX+O and OVX+E animals (see text).
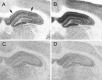
Fig. 5.
Representative autoradiograms of total [3H]glutamate binding in the hippocampus of ovariectomized rats treated with sesame oil (A) or estradiol (B) and the [3H]glutamate binding that remains after displacement with NMDA in OVX+O (C) or OVX+E (D) animals. Agonist binding to the NMDA receptor was taken to be the amount of total glutamate binding that was displaced by NMDA, i.e., the difference between the top and bottom panels. Note that total glutamate binding is increased by estradiol treatment and that this difference is attributable to enhanced NMDA binding because there is no difference between OVX+O and OVX+E in the binding that remains after displacement with NMDA. NMDA-displaceable [3H]glutamate binding within the CA1 region (indicated by arrows) is quantified in Figure6_A_.
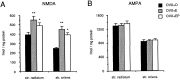
Fig. 6.
Bar graphs depicting the effect of estradiol and progesterone treatments on NMDA-displaceable [3H]glutamate (A) and [3H]AMPA (B) binding in the regions of the hippocampus containing the apical (str. radiatum) or basal (str. oriens) dendrites of CA1 pyramidal cells. Filled bars represent binding in OVX+O animals; _stippled bars_represent binding in OVX+E animals; open bars represent binding in ovariectomized animals treated with both estradiol and progesterone (OVX+EP). Note that treatment with either estradiol or estradiol plus progesterone increases NMDA receptor binding with no effect on AMPA receptor binding. **, Significant difference from OVX+O (p < 0.01); *, significant difference from OVX+O (p < 0.05).
Fig. 7.
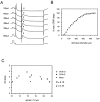
A shows representative intracellular recordings from a CA1 pyramidal cell recorded in standard ACSF. Calibration pulses in top (voltage) _traces_indicate 10 mV, 10 msec, in bottom (current)trace 1.0 nA, 10 msec. Stimuli of increasing intensity (indicated at the left of each trace) were delivered to the CA1 st. radiatum embedded in a 100 msec, 0.5 nA hyperpolarizing current pulse. EPSP (indicated by arrowheads in A) slope was plotted versus stimulus intensity to generate input/output curves (B). The slope of such intracellular input/output curves was taken as a measure of sensitivity to synaptic input. No correlation between input/output curve slope and dendritic spine density was observed under standard recording conditions (C).
Fig. 8.
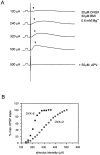
A shows representative intracellular recordings of NMDA receptor-mediated EPSPs (indicated by_arrowheads_) from a CA1 pyramidal cell. Calibration pulse, 10 mV, 10 msec. Stimuli of increasing intensity were delivered to the st. radiatum to generate input/output curves (B). Stimulus intensities are indicated at the left of the each trace. NMDA receptor-mediated EPSPs were recorded with the following modifications of the recording medium: 30 μ
m
CNQX, 30 μ
m
bicuculline (BMI), Mg2+ reduced to 0.6 m
m
; in addition, recorded cells were depolarized to approximately −40 mV and 200 m
m
QX-314 was included in the recording electrode to eliminate Na+ action potentials in the recorded cell. EPSPs generated under these conditions were blocked by addition of 50 μ
m
APV to the recording medium.B shows representative input/output curves from cells in slices from OVX+O and OVX+E animals. Note that the slope of the input/output curve generated from NMDA receptor-mediated EPSPs is greater in the OVX+E cell.
Fig. 9.
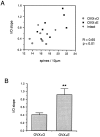
A shows the correlation between the slope of input/output curves generated from NMDA receptor-mediated CA1 pyramidal cell EPSPs and dendritic spine density on each cell. Data from cells in OVX+O (open squares), OVX+E (filled squares), and gonadally intact (open circles) animals are plotted together. B is a bar graph depicting the mean slope of input/output curves generated from NMDA receptor-mediated EPSPs in cells from OVX+O compared to OVX+E animals. Note that the mean slope of the input/output curves generated from NMDA receptor-mediated EPSPs is significantly greater in the cells from OVX+E animals; ** p < 0.01.
Similar articles
- Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices.
Pozzo-Miller LD, Inoue T, Murphy DD. Pozzo-Miller LD, et al. J Neurophysiol. 1999 Mar;81(3):1404-11. doi: 10.1152/jn.1999.81.3.1404. J Neurophysiol. 1999. PMID: 10085365 - Blocking GABA(A) inhibition reveals AMPA- and NMDA-receptor-mediated polysynaptic responses in the CA1 region of the rat hippocampus.
Crépel V, Khazipov R, Ben-Ari Y. Crépel V, et al. J Neurophysiol. 1997 Apr;77(4):2071-82. doi: 10.1152/jn.1997.77.4.2071. J Neurophysiol. 1997. PMID: 9114256 - Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat.
Rudick CN, Woolley CS. Rudick CN, et al. J Neurosci. 2001 Sep 1;21(17):6532-43. doi: 10.1523/JNEUROSCI.21-17-06532.2001. J Neurosci. 2001. PMID: 11517242 Free PMC article. - Membrane properties and synaptic currents evoked in CA1 interneuron subtypes in rat hippocampal slices.
Morin F, Beaulieu C, Lacaille JC. Morin F, et al. J Neurophysiol. 1996 Jul;76(1):1-16. doi: 10.1152/jn.1996.76.1.1. J Neurophysiol. 1996. PMID: 8836204
Cited by
- Astrocytic glutamate transport is essential for the memory-enhancing effects of 17β-estradiol in ovariectomized mice.
Taxier LR, Pillerová M, Branyan TE, Sohrabji F, Frick KM. Taxier LR, et al. Horm Behav. 2024 Sep;165:105618. doi: 10.1016/j.yhbeh.2024.105618. Epub 2024 Aug 23. Horm Behav. 2024. PMID: 39180889 - The estrous cycle modulates hippocampal spine dynamics, dendritic processing, and spatial coding.
Wolcott NS, Redman WT, Karpinska M, Jacobs EG, Goard MJ. Wolcott NS, et al. bioRxiv [Preprint]. 2024 Aug 3:2024.08.02.606418. doi: 10.1101/2024.08.02.606418. bioRxiv. 2024. PMID: 39131375 Free PMC article. Preprint. - Low testosterone levels relate to poorer cognitive function in women in an APOE-ε4-dependant manner.
Dratva MA, Banks SJ, Panizzon MS, Galasko D, Sundermann EE; Alzheimer’s Disease Neuroimaging Initiative. Dratva MA, et al. Biol Sex Differ. 2024 Jun 5;15(1):45. doi: 10.1186/s13293-024-00620-4. Biol Sex Differ. 2024. PMID: 38835072 Free PMC article. - Peri-ictal activation of dorsomedial dorsal raphe serotonin neurons reduces mortality associated with maximal electroshock seizures.
Petrucci AN, Jones AR, Kreitlow BL, Buchanan GF. Petrucci AN, et al. Brain Commun. 2024 Mar 14;6(2):fcae052. doi: 10.1093/braincomms/fcae052. eCollection 2024. Brain Commun. 2024. PMID: 38487550 Free PMC article. - G protein-coupled estrogen receptor (GPER) in the dorsal hippocampus regulates memory consolidation in gonadectomized male mice, likely via different signaling mechanisms than in female mice.
Machado GDB, Schnitzler AL, Fleischer AW, Beamish SB, Frick KM. Machado GDB, et al. Horm Behav. 2024 May;161:105516. doi: 10.1016/j.yhbeh.2024.105516. Epub 2024 Mar 1. Horm Behav. 2024. PMID: 38428223 Free PMC article.
References
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
- Buterbaugh GG, Hudson GM. Estradiol replacement to female rats facilitates dorsal hippocampal but not ventral hippocampal kindled seizure acquisition. Exp Neurol. 1991;111:55–64. - PubMed
- Dewar D, Chalmers DT, Graham DI, McCulloch J. Glutamate metabotropic and AMPA binding sites are reduced in Alzheimer’s disease: an autoradiographic study of the hippocampus. Brain Res. 1991;553:58–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous