Behavioral effects of estrogen receptor gene disruption in male mice - PubMed (original) (raw)
Behavioral effects of estrogen receptor gene disruption in male mice
S Ogawa et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Gonadal steroid hormones regulate sexually dimorphic development of brain functions and behaviors. Their nuclear receptors offer the opportunity to relate molecular events in neurons to simple instinctive mammalian behaviors. We have determined the role of estrogen receptor (ER) activation by endogenous estrogen in the development of male-typical behaviors by the use of transgenic estrogen-receptor-deficient (ERKO) mice. Surprisingly, in spite of the fact that they are infertile, ERKO mice showed normal motivation to mount females but they achieved less intromissions and virtually no ejaculations. Aggressive behaviors were dramatically reduced and male-typical offensive attacks were rarely displayed by ERKO males. Moreover, ER gene disruption demasculinized open-field behaviors. In the brain, despite the evident loss of functional ER protein, the androgen-dependent system appears to be normally present in ERKO mice. Together, these findings indicate that ER gene expression during development plays a major role in the organization of male-typical aggressive and emotional behaviors in addition to simple sexual behaviors.
Figures
Figure 1
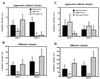
Effects of ER gene disruption on male sexual behaviors. During 30-min sexual behavior tests, there were no differences in mean number of mounts (A) and mean latency to the first mounts (B; which included only the mice that showed the behavior) between three genotypes. In contrast, there were overall genotype differences in mean number of intromissions [C; F(2,49) = 3.930, P < 0.05] and ERKO mice showed significantly fewer intromissions compared with HZ, but not with WT, mice at α = 0.05 (∗). Mean latency to the first intromissions (D; which included only the mice that showed the behavior) of ERKO mice was significantly longer compared with both WT and HZ mice in Test 1 [F(2,15) = 11.473, P < 0.001; ∗∗, significantly different from both WT and HZ at α = 0.05], but not in Test 2. Temporal changes of mean number of mounts (E), mean number of intromissions (F), and percentage of mice ejaculated (G) during 3-hr tests were analyzed for six time blocks of 30 min each. ERKO mice showed intromissions continuously during the entire 3-hr test period but did not ejaculate, whereas WT mice showed a high number of intromissions (∗, P < 0.05) and ejaculated in the first 30 min. Total numbers of mounts (WT vs ERKO; mean ± SEM, 13.92 ± 5.60 vs. 14.82 ± 5.49) and intromissions (20.08 ± 5.87 vs. 19.00 ± 8.53) during the 3-hr test period were not different between WT and ERKO mice. Vertical bars represent SEM.
Figure 2
Effects of ER gene disruption on aggressive behaviors tested in two different paradigms, resident–intruder tests (A and B) and homogeneous set tests (C and D). Cumulative durations of aggressive bouts with (B and D) and without (A and C) offensive attacks are shown separately with different scales. ERKO mice showed very few aggressive behaviors even without attacks in resident–intruder tests (A; ∗, significantly different from WT mice at α = 0.05), whereas in the homogeneous set tests there were no differences between three genotypes (C). On the other hand, in both tests, cumulative duration of offensive attacks (B and D) were greatly reduced in ERKO mice compared with both WT and HZ mice (∗∗, α = 0.05).
Figure 3
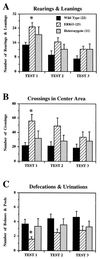
Effects of ER gene disruption on open-field behaviors in 5-min tests over 3 consecutive days. There were overall significant genotype differences in the mean number of rearings and leanings [A; F(2,53) = 3.731, P < 0.05], mean number of crossings in the center area [B; F(2,53) = 3.840, P < 0.05], and mean number of defecations and urinations [C; F(2,53) = 3.828, P < 0.05]. ERKO mice showed female-type open-field behaviors compared with WT mice (∗, significantly different from WT, but not HZ, mice at α = 0.05), i.e., higher number of rearings and leanings, entered more often to the center area, and defecated less. In these three measurements, there was also a significant day effect but no interaction with genotype: in all three genotype groups, the number of rearings/leanings and the number of crossings in the center area deceased, and the number of defecations/urinations increased over the 3 days of tests.
Figure 4
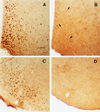
Photomicrographs showing the ER–IR cells (stained with polyclonal ER21 antibody) in the medial preoptic area and the ventromedial nucleus of hypothalamus of WT (A and C) and ERKO (B and D) mouse brains. The number of ER–IR cells was greatly reduced in ERKO mouse brains but not completely eliminated in the medial preoptic area as indicated with arrows in B. (Scale bar = 75 μm.) Despite the evident loss of ER–IR cells, there was no overall difference on the distribution of AR–IR cells between ERKO and WT mice (see Table 1).
Similar articles
- Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO).
Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Ogawa S, et al. Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14737-41. doi: 10.1073/pnas.250473597. Proc Natl Acad Sci U S A. 2000. PMID: 11114183 Free PMC article. - Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice.
Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Ogawa S, et al. Endocrinology. 1998 Dec;139(12):5058-69. doi: 10.1210/endo.139.12.6358. Endocrinology. 1998. PMID: 9832445 - Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice.
Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Ogawa S, et al. Endocrinology. 1998 Dec;139(12):5070-81. doi: 10.1210/endo.139.12.6357. Endocrinology. 1998. PMID: 9832446 - Estrogen receptor null mice: what have we learned and where will they lead us?
Couse JF, Korach KS. Couse JF, et al. Endocr Rev. 1999 Jun;20(3):358-417. doi: 10.1210/edrv.20.3.0370. Endocr Rev. 1999. PMID: 10368776 Review. - Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes.
Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP. Korach KS, et al. Recent Prog Horm Res. 1996;51:159-86; discussion 186-8. Recent Prog Horm Res. 1996. PMID: 8701078 Review.
Cited by
- Sexually selected traits: a fundamental framework for studies on behavioral epigenetics.
Jašarević E, Geary DC, Rosenfeld CS. Jašarević E, et al. ILAR J. 2012;53(3-4):253-69. doi: 10.1093/ilar.53.3-4.253. ILAR J. 2012. PMID: 23744965 Free PMC article. Review. - Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines.
Gillies GE, McArthur S. Gillies GE, et al. Pharmacol Rev. 2010 Jun;62(2):155-98. doi: 10.1124/pr.109.002071. Epub 2010 Apr 14. Pharmacol Rev. 2010. PMID: 20392807 Free PMC article. Review. - Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO).
Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Ogawa S, et al. Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14737-41. doi: 10.1073/pnas.250473597. Proc Natl Acad Sci U S A. 2000. PMID: 11114183 Free PMC article. - Application of Transgenic Zebrafish Models for Studying the Effects of Estrogenic Endocrine Disrupting Chemicals on Embryonic Brain Development.
Takesono A, Kudoh T, Tyler CR. Takesono A, et al. Front Pharmacol. 2022 Feb 11;13:718072. doi: 10.3389/fphar.2022.718072. eCollection 2022. Front Pharmacol. 2022. PMID: 35264948 Free PMC article. Review. - Sex differences in the neural circuit that mediates female sexual receptivity.
Flanagan-Cato LM. Flanagan-Cato LM. Front Neuroendocrinol. 2011 Apr;32(2):124-36. doi: 10.1016/j.yfrne.2011.02.008. Epub 2011 Feb 19. Front Neuroendocrinol. 2011. PMID: 21338620 Free PMC article. Review.
References
- Meisel R L, Sachs B D. In: Physiology of Reproduction. 2nd Ed. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 3–105.
- Olsen K L. In: Sexual Differentiation. Arnold G A, Howard M, Ingeborg W L, editors. New York: Plenum; 1992. pp. 1–40.
- Simon N G, Whalen R E. Aggressive Behav. 1986;12:255–266.
- Brain P F, Haug M, Kamis A B. In: Hormones and Behavior in Higher Vertebrates. Balthazart J, Prove E, Gilles R, editors. Berlin: Springer; 1983. pp. 290–304.
- Nyby J, Matochik J A, Barfield R J. Horm Behav. 1992;26:24–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases