Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments - PubMed (original) (raw)
Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments
Y W Peng et al. Proc Natl Acad Sci U S A. 1997.
Abstract
Phototransduction in retinal rods involves a G protein-coupled signaling cascade that leads to cGMP hydrolysis and the closure of cGMP-gated cation channels that are open in darkness, producing a membrane hyperpolarization as the light response. For many years there have also been reports of the presence of a phosphoinositide pathway in the rod outer segment, though its functions and the molecular identities of its components are still unclear. Using immunocytochemistry with antibodies against various phosphoinositide-specific phospholipase C (PLC) isozymes (beta1-4, gamma1-2, and delta1-2), we have found PLCbeta4-like immunoreactivity in rod outer segments. Similar experiments with antibodies against the alpha-subunits of the G(q) family of G proteins, which are known to activate PLCbeta4, have also demonstrated G(alpha11)-like immunoreactivity in this location. Immunoblots of total proteins from whole retina or partially purified rod outer segments with anti-PLCbeta4 and anti-G(alpha11) antibodies gave, respectively, a single protein band of the expected molecular mass, suggesting specific labelings. The retinal locations of the two proteins were also supported by in situ hybridization experiments on mouse retina with probes specific for the corresponding mouse genes. These two proteins, or immunologically identical isoforms, therefore likely mediate the phosphoinositide signaling pathway in the rod outer segment. At present, G(alpha11) or a G(alpha11)-like protein represents the only G protein besides transducin (which mediates phototransduction) identified so far in the rod outer segment. Although absent in the outer segment layer, other PLC isoforms as well as G(alpha q) (another G(q) family member), are present elsewhere in the retina.
Figures
Figure 1
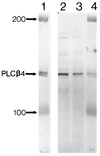
Immunoblotting of purified PLCβ4 from bovine retina (lanes 1 and 4) and HPLC fractions from rat uterus that show high phospholipase C activity (lanes 2 and 3), using the Ab2 antibody. The numbers indicate protein markers of molecular masses 100 and 200 kDa. See refs. and for the methods of collecting the HPLC fractions and assaying phospholipase C activity.
Figure 2
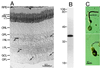
(A) Immunostaining of a cross-section of the bovine retina with an antibody against PLCβ4. (Nomarski differential interference contrast optics; 8-μM frozen section.) The anatomical layers are as follows: RPE, retinal pigment epithelium; PRL, photoreceptor layer containing predominantly the outer segments (ROS) and inner segments (RIS) of rods, though cones are also present in far fewer numbers; ONL, outer nuclear layer containing the cell bodies of the photoreceptors; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; OFL, optic fiber layer. (B) Immunoblotting of total bovine retinal proteins with the anti-PLCβ4 antibody. Sizes of molecular mass standards (×10−3) are shown adjacent to the lane. (C) Dissociated rod (Left) and cone (Right) cells stained with the same antibody. Only the rod outer segment (ROS) shows staining, but apparently not the cone outer segment (COS).
Figure 3
(A) Immunostaining of a cross-section of the bovine retina with an antibody against Gα11. (Nomarski optics; 8-μM frozen section.) The anatomical layers are as in Fig. 2. The arrows indicate blood vessels. (B) Immunoblotting of total bovine retinal proteins with the anti-Gα11 antibody. Sizes of molecular mass standards (×10−3) are shown adjacent to the lane. (C) Dissociated rod (Top) and cone (Bottom) cells stained with the same antibody. Again, only the rod outer segment (ROS), but apparently not the cone outer segment (COS), shows staining.
Figure 4
Immunoblotting of total proteins from partially purified bovine rod outer segments. (A) With the anti-PLCβ4 antibody. (B) With the anti-Gα11 antibody. (C) Experiment examining the degree of purity of the partially purified rod outer segment preparation. Lane 1, partially purified rod outer segments; lane 2, total retinal proteins. Both are stained with the PMc1D1 antibody against the rod cGMP-gated channel, known to be present predominantly in the rod outer segment (38). Densitometric measurements indicated that the intensity of staining was approximately 10:1 between lanes 1 and 2. (D) Experiment and display similar to C, but stained with an antibody against PLCδ2, which is predominantly in Müller cells rather than rod outer segments (see Results). Sizes of molecular mass standards (×10−3) are shown adjacent to the lanes.
Figure 5
In situ hybridization of mouse retinal sections with digoxygenin-labeled riboprobes. The anatomical layers are as in Fig. 2. (A) Bright-field optics; with antisense riboprobe for PLCβ4. (B_–_D) Nomarski optics; 8-μM frozen sections. (B) With sense riboprobe for PLCβ4. (C) With antisense riboprobe for Gα11. (D) With sense riboprobe for Gα11.
Figure 6
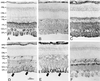
Immunostaining of bovine retinal cross-sections for other PLC isozymes and also Gαq. (Nomarski optics; 8-μM frozen sections.) The anatomical layers are as in Fig. 2. (A) PLCβ1. (B) PLCβ3. (C) PLCγ1. (D) PLCδ1 (thick arrows indicate stained endfeet of Müller glial cells; arrowheads indicate blood vessels). (E) PLCδ2 (arrows indicate stained endfeet of Müller glial cells). (F) Gαq (arrows indicate stained bipolar cells).
Similar articles
- Phospholipase Cgamma1 in bovine rod outer segments: immunolocalization and light-dependent binding to membranes.
Ghalayini AJ, Weber NR, Rundle DR, Koutz CA, Lambert D, Guo XX, Anderson RE. Ghalayini AJ, et al. J Neurochem. 1998 Jan;70(1):171-8. doi: 10.1046/j.1471-4159.1998.70010171.x. J Neurochem. 1998. PMID: 9422360 - Identification and immunolocalization of phospholipase C in bovine rod outer segments.
Ghalayini AJ, Tarver AP, Mackin WM, Koutz CA, Anderson RE. Ghalayini AJ, et al. J Neurochem. 1991 Oct;57(4):1405-12. doi: 10.1111/j.1471-4159.1991.tb08307.x. J Neurochem. 1991. PMID: 1895111 - Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain.
Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Nakamura M, et al. Eur J Neurosci. 2004 Dec;20(11):2929-44. doi: 10.1111/j.1460-9568.2004.03768.x. Eur J Neurosci. 2004. PMID: 15579147 - Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.
Korenbrot JI, Rebrik TI. Korenbrot JI, et al. Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review. - Regulation of phosphoinositide-specific phospholipase C.
Rhee SG. Rhee SG. Annu Rev Biochem. 2001;70:281-312. doi: 10.1146/annurev.biochem.70.1.281. Annu Rev Biochem. 2001. PMID: 11395409 Free PMC article. Review.
Cited by
- Mislocalized opsin and cAMP signaling: a mechanism for sprouting by rod cells in retinal degeneration.
Wang J, Zhang N, Beuve A, Townes-Anderson E. Wang J, et al. Invest Ophthalmol Vis Sci. 2012 Sep 19;53(10):6355-69. doi: 10.1167/iovs.12-10180. Invest Ophthalmol Vis Sci. 2012. PMID: 22899763 Free PMC article. - Do phosphatidylinositides modulate vertebrate phototransduction?
Womack KB, Gordon SE, He F, Wensel TG, Lu CC, Hilgemann DW. Womack KB, et al. J Neurosci. 2000 Apr 15;20(8):2792-9. doi: 10.1523/JNEUROSCI.20-08-02792.2000. J Neurosci. 2000. PMID: 10751430 Free PMC article. - Activation of phospholipase C mimics the phase shifting effects of light on melatonin rhythms in retinal photoreceptors.
Semple-Rowland S, Madorsky I, Bolch S, Berry J, Smith WC. Semple-Rowland S, et al. PLoS One. 2013 Dec 26;8(12):e83378. doi: 10.1371/journal.pone.0083378. eCollection 2013. PLoS One. 2013. PMID: 24386190 Free PMC article. - Phospholipase C β4 promotes RANKL-dependent osteoclastogenesis by interacting with MKK3 and p38 MAPK.
Lee DK, Jin X, Choi PR, Cui Y, Che X, Lee S, Hur K, Kim HJ, Choi JY. Lee DK, et al. Exp Mol Med. 2025 Feb;57(2):323-334. doi: 10.1038/s12276-025-01390-8. Epub 2025 Feb 3. Exp Mol Med. 2025. PMID: 39894822 Free PMC article. - Phosphoinositides and photoreceptors.
Brockerhoff SE. Brockerhoff SE. Mol Neurobiol. 2011 Dec;44(3):420-5. doi: 10.1007/s12035-011-8208-y. Epub 2011 Sep 18. Mol Neurobiol. 2011. PMID: 21928087 Free PMC article. Review.
References
- Yau K-W. Invest Ophthalmol Visual Sci. 1993;35:9–32. - PubMed
- Ghalayini A J, Anderson R E. Biochem Biophys Res Commun. 1984;124:503–506. - PubMed
- Hayashi F, Amakawa T. Biochem Biophys Res Commun. 1985;128:954–959. - PubMed
- Brown J E, Blazynski C, Cohen A I. Biochem Biophys Res Comm. 1987;146:1392–1396. - PubMed
- Millar F A, Fisher S C, Muir C A, Edwards E, Hawthorne J N. Biochim Biophys Acta. 1988;970:205–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources