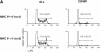Quantitative impact of thymic clonal deletion on the T cell repertoire - PubMed (original) (raw)
Quantitative impact of thymic clonal deletion on the T cell repertoire
J P van Meerwijk et al. J Exp Med. 1997.
Abstract
Interactions between major histocompatibility complex (MHC) molecules expressed on stromal cells and antigen-specific receptors on T cells shape the repertoire of mature T lymphocytes emerging from the thymus. Some thymocytes with appropriate receptors are stimulated to undergo differentiation to the fully mature state (positive selection), whereas others with strongly autoreactive receptors are triggered to undergo programmed cell death before completing this differentiation process (negative selection). The quantitative impact of negative selection on the potentially available repertoire is currently unknown. To address this issue, we have constructed radiation bone marrow chimeras in which MHC molecules are present on radioresistant thymic epithelial cells (to allow positive selection) but absent from radiosensitive hematopoietic elements responsible for negative selection. In such chimeras, the number of mature thymocytes was increased by twofold as compared with appropriate control chimeras This increase in steady-state numbers of mature thymocytes was not related to proliferation, increased retention, or recirculation and was accompanied by a similar two- to threefold increase in the de novo rate of generation of mature cells. Taken together, our data indicate that half to two-thirds of the thymocytes able to undergo positive selection die before full maturation due to negative selection.
Figures
Figure 1
Positive selection depends on expression of MHC molecules on radioresistant thymic epithelial cells. (A) Thymi from wild-type→ wild-type, MHC I°II°→ I°II°, and wild-type→ I°II° chimeras were analyzed by flow cytometry 6 wk after grafting. Thymocytes were stained with anti-CD8, anti-TCR, and anti-CD4 antibodies and analyzed on a FACScan® using LYSYS II software. Contour plots are 75% logarithmic and TCR histograms are from total thymus and from electronically gated cells using the gates indicated in the figure. (B) Percentages of CD4SP (CD4+CD8− TCRhigh) and CD8SP cells (CD4−CD8+TCRhigh) in thymi from the indicated chimeras were determined and depicted as percentage of an age- and sex-matched nonchimeric control mouse ± SD.
Figure 1
Positive selection depends on expression of MHC molecules on radioresistant thymic epithelial cells. (A) Thymi from wild-type→ wild-type, MHC I°II°→ I°II°, and wild-type→ I°II° chimeras were analyzed by flow cytometry 6 wk after grafting. Thymocytes were stained with anti-CD8, anti-TCR, and anti-CD4 antibodies and analyzed on a FACScan® using LYSYS II software. Contour plots are 75% logarithmic and TCR histograms are from total thymus and from electronically gated cells using the gates indicated in the figure. (B) Percentages of CD4SP (CD4+CD8− TCRhigh) and CD8SP cells (CD4−CD8+TCRhigh) in thymi from the indicated chimeras were determined and depicted as percentage of an age- and sex-matched nonchimeric control mouse ± SD.
Figure 2
Increased CD4SP (CD4+CD8−TCRhigh) thymocytes in chimeras lacking MHC class II expression on hematopoietic elements. Groups of sex- and age-matched chimeras were analyzed on the same day 6–8 wk after engraftment. Flow cytometry was performed using antiTCR, anti-CD4, and anti-CD8 antibodies. In each experiment, the ratio of CD4SP cells in the indicated groups was calculated. Error bars indicate SD. The increased ratio of CD4SP cells in MHC II°→ MHC I° versus MHC II+→ MHC I° chimeras is statistically significant as assessed by the Student's t test (P <0.0001), whereas the ratio of CD4SP thymocytes in MHC I°→ MHC I° versus MHC I+→ MHC I° chimeras is not significantly increased (P = 0.02).
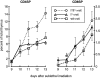
Figure 4
Accelerated kinetics of generation of CD4SP and CD8SP thymocytes in wild-type hosts lacking MHC class II or I, respectively, on hematopoietic elements. 6-wk-old wt→ wt (n = 3, day 9; n = 6, days 10– 13), MHC I°II°→ wt (n = 3 each day), and MHC I°→ wt (n = 3) chimeras were sublethally irradiated (720 rads) and analyzed on day 9–13 as in Fig. 2. Data represent average percentage of CD4+CD8−TCRhigh and CD4−CD8+TCRhigh thymocytes ± SD.
Figure 3
Increase in CD4SP thymocytes in MHC II°→ MHC I° chimeras is not due to (A) proliferation or (B) recirculation of peripheral T lymphocytes. (A) Cell cycle analysis (PI incorporation) was performed on ethanol-fixed total thymocytes and electronically sorted CD4+CD8− TCRhigh cells (purity ⩾95%). Representative results are shown. The statistics represent mean percentage cells in S+G2/M phase ± SD from the indicated number of experiments. (B) Four-color flow cytometry was performed using anti-CD4, anti-CD8, and anti-TCR antibodies combined with anti-CD44, anti-HSA, or anti-CD69. The CD44, HSA, and CD69 histograms are of electronically gated CD4+CD8−TCRhigh cells. Representative results are shown. The statistics represent mean percentage ± SD from the indicated number of experiments.
Figure 3
Increase in CD4SP thymocytes in MHC II°→ MHC I° chimeras is not due to (A) proliferation or (B) recirculation of peripheral T lymphocytes. (A) Cell cycle analysis (PI incorporation) was performed on ethanol-fixed total thymocytes and electronically sorted CD4+CD8− TCRhigh cells (purity ⩾95%). Representative results are shown. The statistics represent mean percentage cells in S+G2/M phase ± SD from the indicated number of experiments. (B) Four-color flow cytometry was performed using anti-CD4, anti-CD8, and anti-TCR antibodies combined with anti-CD44, anti-HSA, or anti-CD69. The CD44, HSA, and CD69 histograms are of electronically gated CD4+CD8−TCRhigh cells. Representative results are shown. The statistics represent mean percentage ± SD from the indicated number of experiments.
Similar articles
- Intrathymic maturation of murine T lymphocytes from CD8+ precursors.
Guidos CJ, Weissman IL, Adkins B. Guidos CJ, et al. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7542-6. doi: 10.1073/pnas.86.19.7542. Proc Natl Acad Sci U S A. 1989. PMID: 2508090 Free PMC article. - A unified model for T cell antigen recognition and thymic selection of the T cell repertoire.
Mannie MD. Mannie MD. J Theor Biol. 1991 Jul 21;151(2):169-92. doi: 10.1016/s0022-5193(05)80360-1. J Theor Biol. 1991. PMID: 1943141 - Class II-positive hematopoietic cells cannot mediate positive selection of CD4+ T lymphocytes in class II-deficient mice.
Markowitz JS, Auchincloss H Jr, Grusby MJ, Glimcher LH. Markowitz JS, et al. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2779-83. doi: 10.1073/pnas.90.7.2779. Proc Natl Acad Sci U S A. 1993. PMID: 8464889 Free PMC article. - Role of thymic cortex-specific self-peptides in positive selection of T cells.
Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, Tanaka K. Takahama Y, et al. Semin Immunol. 2010 Oct;22(5):287-93. doi: 10.1016/j.smim.2010.04.012. Epub 2010 May 26. Semin Immunol. 2010. PMID: 20510627 Review. - Negative selection--clearing out the bad apples from the T-cell repertoire.
Palmer E. Palmer E. Nat Rev Immunol. 2003 May;3(5):383-91. doi: 10.1038/nri1085. Nat Rev Immunol. 2003. PMID: 12766760 Review.
Cited by
- Theories and quantification of thymic selection.
Yates AJ. Yates AJ. Front Immunol. 2014 Feb 4;5:13. doi: 10.3389/fimmu.2014.00013. eCollection 2014. Front Immunol. 2014. PMID: 24550908 Free PMC article. Review. - Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus.
Perry JSA, Lio CJ, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Perry JSA, et al. Immunity. 2014 Sep 18;41(3):414-426. doi: 10.1016/j.immuni.2014.08.007. Epub 2014 Sep 11. Immunity. 2014. PMID: 25220213 Free PMC article. - B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells.
Frommer F, Waisman A. Frommer F, et al. PLoS One. 2010 Oct 20;5(10):e15372. doi: 10.1371/journal.pone.0015372. PLoS One. 2010. PMID: 20976010 Free PMC article. - In vivo maintenance of T-lymphocyte unresponsiveness induced by thymic medullary epithelium requires antigen presentation by radioresistant cells.
Hudrisier D, Feau S, Bonnet V, Romagnoli P, Van Meerwijk JP. Hudrisier D, et al. Immunology. 2003 Jan;108(1):24-31. doi: 10.1046/j.1365-2567.2003.01546.x. Immunology. 2003. PMID: 12519299 Free PMC article. - The thymus road to a T cell: migration, selection, and atrophy.
Ruiz Pérez M, Vandenabeele P, Tougaard P. Ruiz Pérez M, et al. Front Immunol. 2024 Aug 27;15:1443910. doi: 10.3389/fimmu.2024.1443910. eCollection 2024. Front Immunol. 2024. PMID: 39257583 Free PMC article. Review.
References
- Swain SL. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. - PubMed
- Anderson G, Moore NC, Owen JJT, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. - PubMed
- Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. - PubMed
- Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+thymocytes selected by MHC class I molecules. Science (Wash DC) 1994;263:1774–1778. - PubMed